NephCure Accelerating Cures Institute: A Multidisciplinary Consortium to Improve Care for Nephrotic Syndrome
- PMID: 29725648
- PMCID: PMC5932133
- DOI: 10.1016/j.ekir.2017.11.016
NephCure Accelerating Cures Institute: A Multidisciplinary Consortium to Improve Care for Nephrotic Syndrome
Abstract
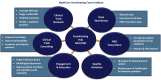
Introduction: NephCure Accelerating Cures Institute (NACI) is a collaborative organization sponsored by NephCure Kidney International and the University of Michigan. The Institute is composed of 7 cores designed to improve treatment options and outcomes for patients with glomerular disease: Clinical Trials Network, Data Warehouse, Patient-Reported Outcomes (PRO) and Endpoints Consortium, Clinical Trials Consulting Team, Quality Initiatives, Education and Engagement, and Data Coordinating Center.
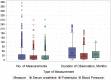
Methods: The Trials Network includes 22 community- and hospital-based nephrology practices, 14 of which are trial-only sites. Eight sites participate in the NACI Registry, and as of October 2017, 1054 patients are enrolled with diagnoses including but not limited to focal segmental glomerulosclerosis, minimal change disease, membranous nephropathy, IgA nephropathy, and childhood-onset nephrotic syndrome. By using electronic health record data extraction, robust and efficient clinical data are captured while minimizing the burden to site-based network staff.
Results: The Data Warehouse includes her-extracted data from registry patients, PRO development data, and data from completed observational studies and clinical trials. The Clinical Trial Consulting Team provides support for trial design in rare diseases leveraging these data. The PRO and Endpoints Consortium develops shorter-term endpoints while capturing the patient-reported significance of interventions under study. The Quality Initiatives and Education/Engagement cores elevate the level of care for patients. The Data Coordinating Center manages the analysis and operations of the Institute.
Conclusion: By engaging with patients, academia, industry, and patient advocate community representatives, including our Patient Advisory Board, NACI strives for better outcomes and treatments using evidence-based support for clinical trial design.
Keywords: clinical trials; electronic health record; glomerular disease; nephrotic syndrome; patient advisory; patient-reported outcomes.
Figures



References
-
- Gipson D.S., Messer K.L., Tran C.L. Inpatient health care utilization in the United States among children, adolescents, and young adults with nephrotic syndrome. Am J Kidney Dis. 2013;61:910–917. - PubMed
-
- Gipson D.S., Massengill S.F., Yao L. Management of childhood onset nephrotic syndrome. Pediatrics. 2009;124:747–757. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

