Secreted Giardia intestinalis cysteine proteases disrupt intestinal epithelial cell junctional complexes and degrade chemokines
- PMID: 29726306
- PMCID: PMC5955458
- DOI: 10.1080/21505594.2018.1451284
Secreted Giardia intestinalis cysteine proteases disrupt intestinal epithelial cell junctional complexes and degrade chemokines
Abstract
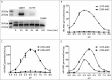
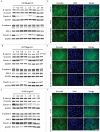
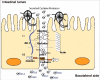
Giardiasis is a common diarrheal disease caused by the protozoan parasite Giardia intestinalis. Cysteine proteases (CPs) are acknowledged as virulence factors in Giardia but their specific role in the molecular pathogenesis of disease is not known. Herein, we aimed to characterize the three main secreted CPs (CP14019, CP16160 and CP16779), which were identified by mass spectrometry in the medium during interaction with intestinal epithelial cells (IECs) in vitro. First, the CPs were epitope-tagged and localized to the endoplasmic reticulum and cytoplasmic vesicle-like structures. Second, we showed that recombinant CPs, expressed in Pichia pastoris, are more active in acidic environment (pH 5.5-6) and we determined the kinetic parameters using fluorogenic substrates. Third, excretory-secretory proteins (ESPs) from Giardia trophozoites affect the localization of apical junctional complex (AJC) proteins and recombinant CPs cleave or re-localize the AJC proteins (claudin-1 and -4, occludin, JAM-1, β-catenin and E-cadherin) of IECs. Finally, we showed that the ESPs and recombinant CPs can degrade several chemokines, including CXCL1, CXCL2, CXCL3, IL-8, CCL2, and CCL20, which are up-regulated in IECs during Giardia-host cell interactions. This is the first study that characterizes the role of specific CPs secreted from Giardia and our results collectively indicate their roles in the disruption of the intestinal epithelial barrier and modulating immune responses during Giardia infections.
Keywords: Host-pathogen interactions; cathepsin B; chemokine; diarrhea; intestinal barrier; parasite; secretion; tight junction.
Figures




Similar articles
-
Cleavage specificity of recombinant Giardia intestinalis cysteine proteases: Degradation of immunoglobulins and defensins.Mol Biochem Parasitol. 2019 Jan;227:29-38. doi: 10.1016/j.molbiopara.2018.10.004. Epub 2018 Nov 17. Mol Biochem Parasitol. 2019. PMID: 30458129
-
Giardia Cysteine Proteases: The Teeth behind the Smile.Trends Parasitol. 2019 Aug;35(8):636-648. doi: 10.1016/j.pt.2019.06.003. Epub 2019 Jul 3. Trends Parasitol. 2019. PMID: 31279655 Review.
-
Characterization of proteolytic activities of Giardia lamblia with the ability to cleave His-tagged N-terminal sequences.Mol Biochem Parasitol. 2019 Mar;228:16-26. doi: 10.1016/j.molbiopara.2019.01.001. Epub 2019 Jan 15. Mol Biochem Parasitol. 2019. PMID: 30658179
-
Characterization of the Giardia intestinalis secretome during interaction with human intestinal epithelial cells: The impact on host cells.PLoS Negl Trop Dis. 2017 Dec 11;11(12):e0006120. doi: 10.1371/journal.pntd.0006120. eCollection 2017 Dec. PLoS Negl Trop Dis. 2017. PMID: 29228011 Free PMC article.
-
Giardia duodenalis: Role of secreted molecules as virulent factors in the cytotoxic effect on epithelial cells.Adv Parasitol. 2019;106:129-169. doi: 10.1016/bs.apar.2019.07.003. Epub 2019 Aug 20. Adv Parasitol. 2019. PMID: 31630757 Review.
Cited by
-
Quantitative proteomic analysis and functional characterization of Acanthamoeba castellanii exosome-like vesicles.Parasit Vectors. 2019 Oct 9;12(1):467. doi: 10.1186/s13071-019-3725-z. Parasit Vectors. 2019. PMID: 31597577 Free PMC article.
-
Recent insights into innate and adaptive immune responses to Giardia.Adv Parasitol. 2019;106:171-208. doi: 10.1016/bs.apar.2019.07.004. Epub 2019 Aug 1. Adv Parasitol. 2019. PMID: 31630758 Free PMC article. Review.
-
Protozoa-Derived Extracellular Vesicles on Intercellular Communication with Special Emphasis on Giardia lamblia.Microorganisms. 2022 Dec 7;10(12):2422. doi: 10.3390/microorganisms10122422. Microorganisms. 2022. PMID: 36557675 Free PMC article. Review.
-
Regulatory Functions of Hypoxia in Host-Parasite Interactions: A Focus on Enteric, Tissue, and Blood Protozoa.Microorganisms. 2023 Jun 16;11(6):1598. doi: 10.3390/microorganisms11061598. Microorganisms. 2023. PMID: 37375100 Free PMC article. Review.
-
Giardia duodenalis Induces Apoptosis in Intestinal Epithelial Cells via Reactive Oxygen Species-Mediated Mitochondrial Pathway In Vitro.Pathogens. 2020 Aug 23;9(9):693. doi: 10.3390/pathogens9090693. Pathogens. 2020. PMID: 32842537 Free PMC article.
References
-
- Ankarklev J, Jerlström-Hultqvist J, Ringqvist E, et al.. Behind the smile: Cell biology and disease mechanisms of Giardia species. Nat Rev microbiol. 2010;8:413–22. - PubMed
-
- Certad G, Viscogliosi E, Chabe M, et al.. Pathogenic mechanisms of Cryptosporidium and Giardia. Trends Parasitol. 2017;33:561–76. - PubMed
-
- Dawson SC. An insider's guide to the microtubule cytoskeleton of Giardia. Cell Microbiol. 2010;12:588–98. - PubMed
-
- Einarsson E, Ma'ayeh S, Svard SG. An up-date on Giardia and giardiasis. Cur Opin Microbiol. 2016;34:47–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
