Understanding the role of the perivascular space in cerebral small vessel disease
- PMID: 29726891
- PMCID: PMC6455920
- DOI: 10.1093/cvr/cvy113
Understanding the role of the perivascular space in cerebral small vessel disease
Abstract
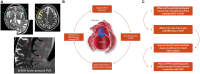
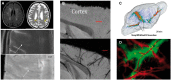
Small vessel diseases (SVDs) are a group of disorders that result from pathological alteration of the small blood vessels in the brain, including the small arteries, capillaries and veins. Of the 35-36 million people that are estimated to suffer from dementia worldwide, up to 65% have an SVD component. Furthermore, SVD causes 20-25% of strokes, worsens outcome after stroke and is a leading cause of disability, cognitive impairment and poor mobility. Yet the underlying cause(s) of SVD are not fully understood. Magnetic resonance imaging has confirmed enlarged perivascular spaces (PVS) as a hallmark feature of SVD. In healthy tissue, these spaces are proposed to form part of a complex brain fluid drainage system which supports interstitial fluid exchange and may also facilitate clearance of waste products from the brain. The pathophysiological signature of PVS and what this infers about their function and interaction with cerebral microcirculation, plus subsequent downstream effects on lesion development in the brain has not been established. Here we discuss the potential of enlarged PVS to be a unique biomarker for SVD and related brain disorders with a vascular component. We propose that widening of PVS suggests presence of peri-vascular cell debris and other waste products that form part of a vicious cycle involving impaired cerebrovascular reactivity, blood-brain barrier dysfunction, perivascular inflammation and ultimately impaired clearance of waste proteins from the interstitial fluid space, leading to accumulation of toxins, hypoxia, and tissue damage. Here, we outline current knowledge, questions and hypotheses regarding understanding the brain fluid dynamics underpinning dementia and stroke through the common denominator of SVD.
Figures
References
-
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689–701. - PubMed
-
- Greenberg SM. Small vessels, big problems. N Engl J Med 2006;354:1451–1453. - PubMed
-
- Munoz DG. Small vessel disease: neuropathology. Int Psychogeriatr 2003;15:67–69. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

