Biphasic zinc compartmentalisation in a human fungal pathogen
- PMID: 29727465
- PMCID: PMC5955600
- DOI: 10.1371/journal.ppat.1007013
Biphasic zinc compartmentalisation in a human fungal pathogen
Abstract
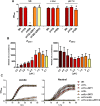
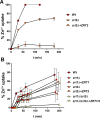
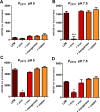
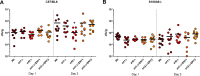
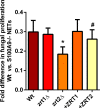
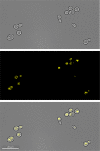
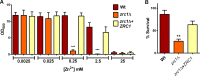
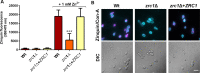
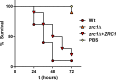
Nutritional immunity describes the host-driven manipulation of essential micronutrients, including iron, zinc and manganese. To withstand nutritional immunity and proliferate within their hosts, pathogenic microbes must express efficient micronutrient uptake and homeostatic systems. Here we have elucidated the pathway of cellular zinc assimilation in the major human fungal pathogen Candida albicans. Bioinformatics analysis identified nine putative zinc transporters: four cytoplasmic-import Zip proteins (Zrt1, Zrt2, Zrt3 and orf19.5428) and five cytoplasmic-export ZnT proteins (orf19.1536/Zrc1, orf19.3874, orf19.3769, orf19.3132 and orf19.52). Only Zrt1 and Zrt2 are predicted to localise to the plasma membrane and here we demonstrate that Zrt2 is essential for C. albicans zinc uptake and growth at acidic pH. In contrast, ZRT1 expression was found to be highly pH-dependent and could support growth of the ZRT2-null strain at pH 7 and above. This regulatory paradigm is analogous to the distantly related pathogenic mould, Aspergillus fumigatus, suggesting that pH-adaptation of zinc transport may be conserved in fungi and we propose that environmental pH has shaped the evolution of zinc import systems in fungi. Deletion of C. albicans ZRT2 reduced kidney fungal burden in wild type, but not in mice lacking the zinc-chelating antimicrobial protein calprotectin. Inhibition of zrt2Δ growth by neutrophil extracellular traps was calprotectin-dependent. This suggests that, within the kidney, C. albicans growth is determined by pathogen-Zrt2 and host-calprotectin. As well as serving as an essential micronutrient, zinc can also be highly toxic and we show that C. albicans deals with this potential threat by rapidly compartmentalising zinc within vesicular stores called zincosomes. In order to understand mechanistically how this process occurs, we created deletion mutants of all five ZnT-type transporters in C. albicans. Here we show that, unlike in Saccharomyces cerevisiae, C. albicans Zrc1 mediates zinc tolerance via zincosomal zinc compartmentalisation. This novel transporter was also essential for virulence and liver colonisation in vivo. In summary, we show that zinc homeostasis in a major human fungal pathogen is a multi-stage process initiated by Zrt1/Zrt2-cellular import, followed by Zrc1-dependent intracellular compartmentalisation.
Conflict of interest statement
The authors have declared that no competing interests exist
Figures
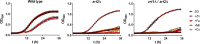




References
-
- Waldron KJ, Rutherford JC, Ford D, Robinson NJ (2009) Metalloproteins and metal sensing. Nature 460: 823–830. doi: 10.1038/nature08300 - DOI - PubMed
-
- Andreini C, Bertini I, Rosato A (2009) Metalloproteomes: a bioinformatic approach. Acc Chem Res 42: 1471–1479. doi: 10.1021/ar900015x - DOI - PubMed
-
- Skaar EP (2010) The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog 6: e1000949 doi: 10.1371/journal.ppat.1000949 - DOI - PMC - PubMed
-
- Hood MI, Skaar EP (2012) Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10: 525–537. doi: 10.1038/nrmicro2836 - DOI - PMC - PubMed
-
- Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charriere GM, Waddell SJ, Foti M, Lugo-Villarino G, Gao Q, Maridonneau-Parini I, Butcher PD, Castagnoli PR, Gicquel B, de Chastellier C, Neyrolles O (2011) Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 10: 248–259. doi: 10.1016/j.chom.2011.08.006 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

