Fasting Activates Fatty Acid Oxidation to Enhance Intestinal Stem Cell Function during Homeostasis and Aging
- PMID: 29727683
- PMCID: PMC5940005
- DOI: 10.1016/j.stem.2018.04.001
Fasting Activates Fatty Acid Oxidation to Enhance Intestinal Stem Cell Function during Homeostasis and Aging
Abstract
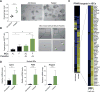
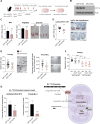
Diet has a profound effect on tissue regeneration in diverse organisms, and low caloric states such as intermittent fasting have beneficial effects on organismal health and age-associated loss of tissue function. The role of adult stem and progenitor cells in responding to short-term fasting and whether such responses improve regeneration are not well studied. Here we show that a 24 hr fast augments intestinal stem cell (ISC) function in young and aged mice by inducing a fatty acid oxidation (FAO) program and that pharmacological activation of this program mimics many effects of fasting. Acute genetic disruption of Cpt1a, the rate-limiting enzyme in FAO, abrogates ISC-enhancing effects of fasting, but long-term Cpt1a deletion decreases ISC numbers and function, implicating a role for FAO in ISC maintenance. These findings highlight a role for FAO in mediating pro-regenerative effects of fasting in intestinal biology, and they may represent a viable strategy for enhancing intestinal regeneration.
Keywords: aging; fasting; fatty acid oxidation; intestinal stem cells; intestine; metabolism; mitochondrial metabolism; stem cells.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors M.M.M., D.M.S. and Ö.H.Y. have filed a patent related to these findings. The authors declare no competing interests.
Figures


Comment in
-
Intestinal Stem Cells Live Off the Fat of the Land.Cell Stem Cell. 2018 May 3;22(5):611-612. doi: 10.1016/j.stem.2018.04.018. Cell Stem Cell. 2018. PMID: 29727673
References
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

