Interaction between a MAPT variant causing frontotemporal dementia and mutant APP affects axonal transport
- PMID: 29729423
- PMCID: PMC5998378
- DOI: 10.1016/j.neurobiolaging.2018.03.033
Interaction between a MAPT variant causing frontotemporal dementia and mutant APP affects axonal transport
Abstract
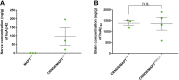
In Alzheimer's disease, many indicators point to a central role for poor axonal transport, but the potential for stimulating axonal transport to alleviate the disease remains largely untested. Previously, we reported enhanced anterograde axonal transport of mitochondria in 8- to 11-month-old MAPTP301L knockin mice, a genetic model of frontotemporal dementia with parkinsonism-17T. In this study, we further characterized the axonal transport of mitochondria in younger MAPTP301L mice crossed with the familial Alzheimer's disease model, TgCRND8, aiming to test whether boosting axonal transport in young TgCRND8 mice can alleviate axonal swelling. We successfully replicated the enhancement of anterograde axonal transport in young MAPTP301L/P301L knockin animals. Surprisingly, we found that in the presence of the amyloid precursor protein mutations, MAPTP301L/P3101L impaired anterograde axonal transport. The numbers of plaque-associated axonal swellings or amyloid plaques in TgCRND8 brains were unaltered. These findings suggest that amyloid-β promotes an action of mutant tau that impairs axonal transport. As amyloid-β levels increase with age even without amyloid precursor protein mutation, we suggest that this rise could contribute to age-related decline in frontotemporal dementia.
Keywords: Alzheimer's disease; Axonal transport; Aβ; FTDP-17T; Mitochondria; P301L mutation.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Adalbert R., Coleman M.P. Review: axon pathology in age-related neurodegenerative disorders. Neuropathol. Appl. Neurobiol. 2013;39:90–108. - PubMed
-
- Adalbert R., Nogradi A., Babetto E., Janeckova L., Walker S.A., Kerschensteiner M., Misgeld T., Coleman M.P. Severely dystrophic axons at amyloid plaques remain continuous and connected to viable cell bodies. Brain. 2009;132(Pt 2):402–416. - PubMed
-
- Adalbert R., Nogradi A., Szabo A., Coleman M.P. The slow Wallerian degeneration gene in vivo protects motor axons but not their cell bodies after avulsion and neonatal axotomy. Eur. J. Neurosci. 2006;24:2163–2168. - PubMed
-
- Allen B., Ingram E., Takao M., Smith M.J., Jakes R., Virdee K., Yoshida H., Holzer M., Craxton M., Emson P.C., Atzori C., Migheli A., Crowther R.A., Ghetti B., Spillantini M.G., Goedert M. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J. Neurosci. 2002;22:9340–9351. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

