iPSC-derived neurons profiling reveals GABAergic circuit disruption and acetylated α-tubulin defect which improves after iHDAC6 treatment in Rett syndrome
- PMID: 29730163
- PMCID: PMC9410763
- DOI: 10.1016/j.yexcr.2018.05.001
iPSC-derived neurons profiling reveals GABAergic circuit disruption and acetylated α-tubulin defect which improves after iHDAC6 treatment in Rett syndrome
Abstract
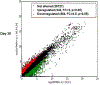
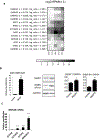
Mutations in MECP2 gene have been identified in more than 95% of patients with classic Rett syndrome, one of the most common neurodevelopmental disorders in females. Taking advantage of the breakthrough technology of genetic reprogramming, we investigated transcriptome changes in neurons differentiated from induced Pluripotent Stem Cells (iPSCs) derived from patients with different mutations. Profiling by RNA-seq in terminally differentiated neurons revealed a prominent GABAergic circuit disruption along with a perturbation of cytoskeleton dynamics. In particular, in mutated neurons we identified a significant decrease of acetylated α-tubulin which can be reverted by treatment with selective inhibitors of HDAC6, the main α-tubulin deacetylase. These findings contribute to shed light on Rett pathogenic mechanisms and provide hints for the treatment of Rett-associated epileptic behavior as well as for the definition of new therapeutic strategies for Rett syndrome.
Keywords: GABA; HDAC6; HDAC6 inhibitors; RNA-seq; acetylated α-tubulin; iPSC‐derived neurons.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interest
Conflicts of interest: none.
Figures


References
-
- Chahrour M, Zoghbi HY, The story of Rett syndrome: from clinic to neurobiology, Neuron 56 (2007) 422–437. - PubMed
-
- Hagberg B, Witt-Engerstrom I, Rett Syndrome: A suggested staging system for describing impairment profile with increasing age towards adolescence, Am J Hum Genet 24 (1986) 47–59. - PubMed
-
- Grillo E, Villard L, Clarke A, Ben Zeev B, Pineda M, Bahi-Buisson N, Hryniewiecka-Jaworska A, Bienvenu T, Armstrong J, Roche-Martinez A, Mari F, Veneselli E, Russo S, Vignoli A, Pini G, Djuric M, Bisgaard AM, Mejaski Bosnjak V, Polgar N, Cogliati F, Ravn K, Pintaudi M, Melegh B, Craiu D, Djukic A, Renieri A, Rett networked database: an integrated clinical and genetic network of Rett syndrome databases, Hum Mutat 33 (2012) 1031–1036. - PubMed
-
- Lyst MJ, Bird A, Rett syndrome: a complex disorder with simple roots, Nat Rev Genet 16 (2015) 261–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

