Genes underlying delayed puberty
- PMID: 29730183
- PMCID: PMC6127442
- DOI: 10.1016/j.mce.2018.05.001
Genes underlying delayed puberty
Abstract
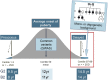
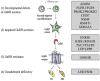
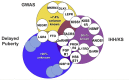
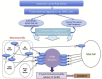
The genetic control of pubertal timing has been a field of active investigation for the last decade, but remains a fascinating and mysterious conundrum. Self-limited delayed puberty (DP), also known as constitutional delay of growth and puberty, represents the extreme end of normal pubertal timing, and is the commonest cause of DP in both boys and girls. Familial self-limited DP has a clear genetic basis. It is a highly heritable condition, which often segregates in an autosomal dominant pattern (with or without complete penetrance) in the majority of families. However, the underlying neuroendocrine pathophysiology and genetic regulation has been largely unknown. Very recently novel gene discoveries from next generation sequencing studies have provided insights into the genetic mutations that lead to familial DP. Further understanding has come from sequencing genes known to cause GnRH deficiency, next generation sequencing studies in patients with early puberty, and from large-scale genome wide association studies in the general population. Results of these studies suggest that the genetic basis of DP is likely to be highly heterogeneous. Abnormalities of GnRH neuronal development, function, and its downstream pathways, metabolic and energy homeostatic derangements, and transcriptional regulation of the hypothalamic-pituitary-gonadal axis may all lead to DP. This variety of different pathogenic mechanisms affecting the release of the puberty 'brake' may take place in several age windows between fetal life and puberty.
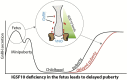
Keywords: Constitutional delay; Delayed puberty; IGSF10; Pubertal timing; Puberty; Puberty genetics; Self-limited delayed puberty.
Copyright © 2018 The Author. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Abitbol L., Zborovski S., Palmert M.R. Evaluation of delayed puberty: what diagnostic tests should be performed in the seemingly otherwise well adolescent? Arch. Dis. Child. 2016;101:767–771. - PubMed
-
- Abreu A.P., Dauber A., Macedo D.B., Noel S.D., Brito V.N., Gill J.C., Cukier P., Thompson I.R., Navarro V.M., Gagliardi P.C., Rodrigues T., Kochi C., Longui C.A., Beckers D., de Zegher F., Montenegro L.R., Mendonca B.B., Carroll R.S., Hirschhorn J.N., Latronico A.C., Kaiser U.B. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N. Engl. J. Med. 2013;368:2467–2475. - PMC - PubMed
-
- Achermann J.C., Gu W.X., Kotlar T.J., Meeks J.J., Sabacan L.P., Seminara S.B., Habiby R.L., Hindmarsh P.C., Bick D.P., Sherins R.J., Crowley W.F., Jr., Layman L.C., Jameson J.L. Mutational analysis of DAX1 in patients with hypogonadotropic hypogonadism or pubertal delay. J. Clin. Endocrinol. Metabol. 1999;84:4497–4500. - PubMed
-
- Aittomaki K., Lucena J.L., Pakarinen P., Sistonen P., Tapanainen J., Gromoll J., Kaskikari R., Sankila E.M., Lehvaslaiho H., Engel A.R., Nieschlag E., Huhtaniemi I., de la Chapelle A. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–968. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

