What is the epidemiology of medication errors, error-related adverse events and risk factors for errors in adults managed in community care contexts? A systematic review of the international literature
- PMID: 29730617
- PMCID: PMC5942474
- DOI: 10.1136/bmjopen-2017-019101
What is the epidemiology of medication errors, error-related adverse events and risk factors for errors in adults managed in community care contexts? A systematic review of the international literature
Erratum in
-
Correction: What is the epidemiology of medication errors, error-related adverse events and risk factors for errors in adults managed in community care contexts? A systematic review of the international literature.BMJ Open. 2019 May 27;9(5):e019101corr1. doi: 10.1136/bmjopen-2017-019101corr1. BMJ Open. 2019. PMID: 31138586 Free PMC article. No abstract available.
Abstract
Objective: To investigate the epidemiology of medication errors and error-related adverse events in adults in primary care, ambulatory care and patients' homes.
Design: Systematic review.
Data source: Six international databases were searched for publications between 1 January 2006 and 31 December 2015.
Data extraction and analysis: Two researchers independently extracted data from eligible studies and assessed the quality of these using established instruments. Synthesis of data was informed by an appreciation of the medicines' management process and the conceptual framework from the International Classification for Patient Safety.
Results: 60 studies met the inclusion criteria, of which 53 studies focused on medication errors, 3 on error-related adverse events and 4 on risk factors only. The prevalence of prescribing errors was reported in 46 studies: prevalence estimates ranged widely from 2% to 94%. Inappropriate prescribing was the most common type of error reported. Only one study reported the prevalence of monitoring errors, finding that incomplete therapeutic/safety laboratory-test monitoring occurred in 73% of patients. The incidence of preventable adverse drug events (ADEs) was estimated as 15/1000 person-years, the prevalence of drug-drug interaction-related adverse drug reactions as 7% and the prevalence of preventable ADE as 0.4%. A number of patient, healthcare professional and medication-related risk factors were identified, including the number of medications used by the patient, increased patient age, the number of comorbidities, use of anticoagulants, cases where more than one physician was involved in patients' care and care being provided by family physicians/general practitioners.
Conclusion: A very wide variation in the medication error and error-related adverse events rates is reported in the studies, this reflecting heterogeneity in the populations studied, study designs employed and outcomes evaluated. This review has identified important limitations and discrepancies in the methodologies used and gaps in the literature on the epidemiology and outcomes of medication errors in community settings.
Keywords: adverse drug events; error-related adverse drug events; incidence; medication errors; prevalence; risk factor.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
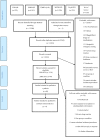

References
-
- Mark S, Little J, Geller S, et al. Principles and practices of medication safety : DiPiro JT TR, Yee GC, Matzke GR, Wells BG, Posey L, Pharmacotherapy: a pathophysiologic approach. New York: McGraw-Hill, 2011.
-
- Krähenbühl-Melcher A, Schlienger R, Lampert M, et al. Drug-related problems in hospitals: a review of the recent literature. Drug Saf 2007;30:379–407. - PubMed
-
- Aitken M, Gorokhovich L. Advancing the responsible use of medicines: applying levers for change. SSRN Electronic Journal 2012. 10.2139/ssrn.2222541 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
