Electron cryomicroscopy as a powerful tool in biomedical research
- PMID: 29730699
- PMCID: PMC5988769
- DOI: 10.1007/s00109-018-1640-y
Electron cryomicroscopy as a powerful tool in biomedical research
Abstract
A human cell is a precisely regulated system that relies on the complex interaction of molecules. Structural insights into the cellular machinery at the atomic level allow us to understand the underlying regulatory mechanism and provide us with a roadmap for the development of novel drugs to fight diseases. Facilitated by recent technological breakthroughs, the Nobel prize-winning technique electron cryomicroscopy (cryo-EM) has become a versatile and extremely powerful tool to solve routinely near-atomic resolution three-dimensional protein structures. Consequently, it has become the focus of attention for structure-based drug design. In this review, we describe the basics of cryo-EM and highlight its growing role in biomedical research. Furthermore, we discuss latest developments as well as future perspectives.
Keywords: Biological macromolecule; Biomedical research; Cryo-EM; Drug design; Electron cryomicroscopy.
Figures
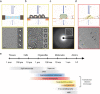


References
-
- Dubochet J, Lepault J, Freeman R, et al. Electron-microscopy of frozen water and aqueous-solutions. J Microsc. 1982;128:219–237. doi: 10.1111/j.1365-2818.1982.tb04625.x. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

