Corticoamygdala Transfer of Socially Derived Information Gates Observational Learning
- PMID: 29731170
- PMCID: PMC6345560
- DOI: 10.1016/j.cell.2018.04.004
Corticoamygdala Transfer of Socially Derived Information Gates Observational Learning
Abstract
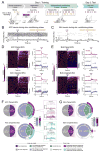
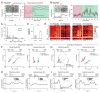
Observational learning is a powerful survival tool allowing individuals to learn about threat-predictive stimuli without directly experiencing the pairing of the predictive cue and punishment. This ability has been linked to the anterior cingulate cortex (ACC) and the basolateral amygdala (BLA). To investigate how information is encoded and transmitted through this circuit, we performed electrophysiological recordings in mice observing a demonstrator mouse undergo associative fear conditioning and found that BLA-projecting ACC (ACC→BLA) neurons preferentially encode socially derived aversive cue information. Inhibition of ACC→BLA alters real-time amygdala representation of the aversive cue during observational conditioning. Selective inhibition of the ACC→BLA projection impaired acquisition, but not expression, of observational fear conditioning. We show that information derived from observation about the aversive value of the cue is transmitted from the ACC to the BLA and that this routing of information is critically instructive for observational fear conditioning. VIDEO ABSTRACT.
Keywords: ChR2; NpHR; amygdala; anterior cingulate cortex; electrophysiology; fear; optogenetics; phototagging; social behavior; state-space.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures





Comment in
-
Watch and learn.Nat Rev Neurosci. 2018 Jul;19(7):388-389. doi: 10.1038/s41583-018-0024-1. Nat Rev Neurosci. 2018. PMID: 29789694 No abstract available.
-
Shining Light on Social Learning Circuits.Trends Cogn Sci. 2018 Aug;22(8):673-675. doi: 10.1016/j.tics.2018.05.002. Epub 2018 May 28. Trends Cogn Sci. 2018. PMID: 29853254
References
-
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. - PubMed
-
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The Anterior Cingulate Cortex. Ann N Y Acad Sci. 2001;935:107–117. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

