Advances in Meningeal Immunity
- PMID: 29731353
- PMCID: PMC6044730
- DOI: 10.1016/j.molmed.2018.04.003
Advances in Meningeal Immunity
Abstract
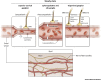
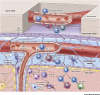
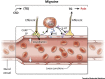
The central nervous system (CNS) is an immunologically specialized tissue protected by a blood-brain barrier. The CNS parenchyma is enveloped by a series of overlapping membranes that are collectively referred to as the meninges. The meninges provide an additional CNS barrier, harbor a diverse array of resident immune cells, and serve as a crucial interface with the periphery. Recent studies have significantly advanced our understanding of meningeal immunity, demonstrating how a complex immune landscape influences CNS functions under steady-state and inflammatory conditions. The location and activation state of meningeal immune cells can profoundly influence CNS homeostasis and contribute to neurological disorders, but these cells are also well equipped to protect the CNS from pathogens. In this review, we discuss advances in our understanding of the meningeal immune repertoire and provide insights into how this CNS barrier operates immunologically under conditions ranging from neurocognition to inflammatory diseases.
Published by Elsevier Ltd.
Figures

References
-
- Coles J.A. Where are we? The anatomy of the murine cortical meninges revisited for intravital imaging, immunology, and clearance of waste from the brain. Prog. Neurobiol. 2017;156:107–148. - PubMed
-
- Protasoni M. The collagenic architecture of human dura mater. J. Neurosurg. 2011;114:1723–1730. - PubMed
-
- Balin B.J. Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J. Comp. Neurol. 1986;251:260–280. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

