Universal Rapid Human Immunodeficiency Virus Screening at Delivery: A Cost-Effectiveness Analysis
- PMID: 29731602
- PMCID: PMC5872626
- DOI: 10.1155/2018/6024698
Universal Rapid Human Immunodeficiency Virus Screening at Delivery: A Cost-Effectiveness Analysis
Abstract
Objective: To determine the cost-effectiveness of universal maternal HIV screening at time of delivery to decrease mother-to-child transmission (MTCT), by comparing the cost and quality-adjusted life years (QALYs) of universal rapid HIV screening at time of delivery to two current standards of care for prenatal HIV screening in the United States.
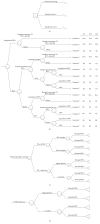
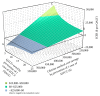
Study design: We conducted a cost-effectiveness analysis to compare the cost and QALY of universal intrapartum rapid HIV screening with two current standards of care: (I) opt-out rapid HIV testing limited to patients without previous third-trimester screening and (II) opt-out rapid HIV testing limited to patients without any prenatal screening. We developed a decision-tree model and performed sensitivity analyses to estimate the impact of variances in QALY, estimated lifetime medical costs, HIV prevalence, and cumulative incidence.
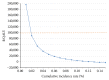
Results: The incremental cost-effectiveness ratio for universal screening was $7,973.45/QALY. The results remained robust to sensitivity analysis, except for annual cumulative incidence. In areas with an annual cumulative incidence rate of <0.02% for reproductive-age women, the incremental cost-effectiveness ratio for the expanded program would exceed $89,926.94/QALY, approaching the commonly applied cost-effectiveness thresholds ($100,000/QALY).
Conclusions: Intrapartum universal rapid HIV screening to decrease MTCT appears cost-effective in populations with high HIV incidence in the United States.
Figures
Similar articles
-
Cost-effectiveness of universal compared with voluntary screening for human immunodeficiency virus among pregnant women in Chicago.Pediatrics. 2000 Apr;105(4):E54. doi: 10.1542/peds.105.4.e54. Pediatrics. 2000. PMID: 10742375
-
Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs.Ann Intern Med. 2006 Dec 5;145(11):797-806. doi: 10.7326/0003-4819-145-11-200612050-00004. Ann Intern Med. 2006. PMID: 17146064
-
Universal Repeat Screening for Human Immunodeficiency Virus in the Third Trimester of Pregnancy: A Cost-Effectiveness Analysis.Obstet Gynecol. 2023 Mar 1;141(3):535-543. doi: 10.1097/AOG.0000000000005086. Obstet Gynecol. 2023. PMID: 36800852
-
Prophylaxis of cervical cancer and related cervical disease: a review of the cost-effectiveness of vaccination against oncogenic HPV types.J Manag Care Pharm. 2010 Apr;16(3):217-30. doi: 10.18553/jmcp.2010.16.3.217. J Manag Care Pharm. 2010. PMID: 20331326 Free PMC article. Review.
-
Risk-Based Breast Cancer Screening versus Population-Based Breast Cancer Screening: A Review of the Comparative Clinical and Cost-Effectiveness [Internet].Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2018 Jan 5. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2018 Jan 5. PMID: 30222280 Free Books & Documents. Review.
Cited by
-
Financing Benefits and Barriers to Routine HIV Screening in Clinical Settings in the United States: A Scoping Review.Int J Environ Res Public Health. 2022 Dec 27;20(1):457. doi: 10.3390/ijerph20010457. Int J Environ Res Public Health. 2022. PMID: 36612775 Free PMC article.
-
Benefits and harms of antenatal and newborn screening programmes in health economic assessments: the VALENTIA systematic review and qualitative investigation.Health Technol Assess. 2024 Jun;28(25):1-180. doi: 10.3310/PYTK6591. Health Technol Assess. 2024. PMID: 38938110 Free PMC article.
References
-
- CDC. Achievements in public health: reduction in perinatal transmission of HIV infection---United States, 1985-2005. Morbidity and Mortality Weekly Report (MMWR) 2006:592–597. - PubMed
-
- ACOG. Prenatal and perinatal human immunodeficiency virus testing: expanded recommendations. Committee Opinion. 2015 - PubMed
-
- Branson B. M., Handsfield H. H., Lampe M. A., Janssen R. S., Taylor A. W., Lyss S. B. MMWR Recommendations and Reports. Vol. 55. Centers for Disease Control and Prevention (CDC); 2006. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings; pp. 1–17. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

