Universal Rapid Human Immunodeficiency Virus Screening at Delivery: A Cost-Effectiveness Analysis
- PMID: 29731602
- PMCID: PMC5872626
- DOI: 10.1155/2018/6024698
Universal Rapid Human Immunodeficiency Virus Screening at Delivery: A Cost-Effectiveness Analysis
Abstract
Objective: To determine the cost-effectiveness of universal maternal HIV screening at time of delivery to decrease mother-to-child transmission (MTCT), by comparing the cost and quality-adjusted life years (QALYs) of universal rapid HIV screening at time of delivery to two current standards of care for prenatal HIV screening in the United States.
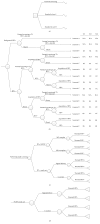
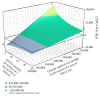
Study design: We conducted a cost-effectiveness analysis to compare the cost and QALY of universal intrapartum rapid HIV screening with two current standards of care: (I) opt-out rapid HIV testing limited to patients without previous third-trimester screening and (II) opt-out rapid HIV testing limited to patients without any prenatal screening. We developed a decision-tree model and performed sensitivity analyses to estimate the impact of variances in QALY, estimated lifetime medical costs, HIV prevalence, and cumulative incidence.
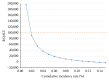
Results: The incremental cost-effectiveness ratio for universal screening was $7,973.45/QALY. The results remained robust to sensitivity analysis, except for annual cumulative incidence. In areas with an annual cumulative incidence rate of <0.02% for reproductive-age women, the incremental cost-effectiveness ratio for the expanded program would exceed $89,926.94/QALY, approaching the commonly applied cost-effectiveness thresholds ($100,000/QALY).
Conclusions: Intrapartum universal rapid HIV screening to decrease MTCT appears cost-effective in populations with high HIV incidence in the United States.
Figures
References
-
- CDC. Achievements in public health: reduction in perinatal transmission of HIV infection---United States, 1985-2005. Morbidity and Mortality Weekly Report (MMWR) 2006:592–597. - PubMed
-
- ACOG. Prenatal and perinatal human immunodeficiency virus testing: expanded recommendations. Committee Opinion. 2015 - PubMed
-
- Branson B. M., Handsfield H. H., Lampe M. A., Janssen R. S., Taylor A. W., Lyss S. B. MMWR Recommendations and Reports. Vol. 55. Centers for Disease Control and Prevention (CDC); 2006. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings; pp. 1–17. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

