Developing and piloting an instrument to prioritize the worries of patients with acute myeloid leukemia
- PMID: 29731612
- PMCID: PMC5927351
- DOI: 10.2147/PPA.S151752
Developing and piloting an instrument to prioritize the worries of patients with acute myeloid leukemia
Abstract
Background: Acute myeloid leukemia (AML) is a rapidly progressing blood cancer for which new treatments are needed. We sought to promote patient-focused drug development (PFDD) for AML by developing and piloting an instrument to prioritize the worries of patients with AML.
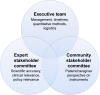
Patients and methods: An innovative community-centered approach was used to engage expert and community stakeholders in the development, pretesting, pilot testing, and dissemination of a novel best-worst scaling instrument. Patient worries were identified through individual interviews (n=15) and group calls. The instrument was developed through rigorous pretesting (n=13) and then piloted among patients and caregivers engaged in this study (n=25). Priorities were assessed using best-worst scores (spanning from +1 to -1) representing the relative number of times that items were endorsed as the most and the least worrying. All findings were presented at a PFDD meeting at the US Food and Drug Administration (FDA) that was attended by >80 stakeholders.
Results: The final instrument included 13 worries spanning issues such as decision making, treatment delivery, physical impacts, and psychosocial effects. Patients and caregivers most prioritized worries about dying from their disease (best minus worst [BW] score=0.73), long-term side effects (BW=0.28), and time in hospital (BW=0.25).
Conclusion: Community-centered approaches are valuable in designing and executing PFDD meetings and associated quantitative surveys to document the experience of patients. Expert and community stakeholders welcomed the opportunity to share their experiences with the FDA and strongly endorsed implementing this survey nationally.
Keywords: acute myeloid leukemia; best–worst scaling; community engagement; patient-focused drug development; stated-preference.
Conflict of interest statement
Disclosure Ernest Voyard and Bernadette O’Donoghue are employees of the LLS. The other authors report no conflicts of interest in this work.
Figures
References
-
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2014. Bethesda, MD, USA: National Cancer Institute; [Accessed February 28, 2018]. Available from: http://seer.cancer.gov/csr/1975_2013/
-
- Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. - PubMed
-
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the world health organization (WHO) classification of myeloid neoplasms in acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. - PubMed
-
- Dohner H, Estey EH, Amadori A, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical