Methyl-β-cyclodextrin quaternary ammonium chitosan conjugate: nanoparticles vs macromolecular soluble complex
- PMID: 29731628
- PMCID: PMC5923277
- DOI: 10.2147/IJN.S160987
Methyl-β-cyclodextrin quaternary ammonium chitosan conjugate: nanoparticles vs macromolecular soluble complex
Abstract
Purpose: The present study aimed to compare a novel cyclodextrin-polymer-drug complex in solution with a dispersed supramolecular nanosize system, made of the same complex, for ability to carry dexamethasone (DEX) across excised rat intestine.
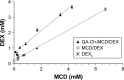
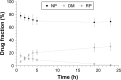
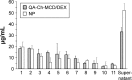
Results: Methyl-β-cyclodextrin-quaternary ammonium chitosan conjugate (QA-Ch-MCD) was obtained by covalent grafting through a 10-atom spacer. The conjugate was characterized by 1H-NMR, resulting in 24.4% w/w of MCD content. Phase solubility profile analysis of the QA-Ch-MCD/DEX complex yielded an association constant of 14037 M-1, vs 4428 M-1 for the plain MCD/DEX complex. Nanoparticle (NP) dispersions resulted from ionotropic gelation of the QA-Ch-MCD/DEX complex by sodium tripolyphosphate, leading to 9.9%±1.4% drug loading efficiency. The mean diameter and zeta potential for NP were 299±32 nm (polydispersity index [PI] 0.049) and 11.5±1.1 mV, respectively. Those for QA-Ch-MCD/DEX were 2.7±0.4 nm (PI 0.048) and 6.7±0.6 mV. QA-Ch-MCD/DEX solutions and corresponding NP dispersions were compared in vitro for water-assisted transport through mucus, DEX permeation through excised rat intestine, and ex vivo mucoadhesivity. The complex showed higher mucoadhesion and lower transport rate through mucus; also, it provided faster drug permeation across excised rat intestine.
Conclusion: Carrier adhesion to mucus surface has played a most important role in favoring transepithelial permeation. Then, within the concerns of the present study, the use of NP seems not to provide any determinant advantage over using the simpler macromolecular complex.
Keywords: chitosan derivatives; cyclodextrin chitosan conjugates; dexamethasone cyclodextrin complex; mucoadhesive nanoparticles; mucoadhesive polymers.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



Similar articles
-
Impact of mucoadhesive polymeric nanoparticulate systems on oral bioavailability of a macromolecular model drug.Eur J Pharm Biopharm. 2018 Sep;130:281-289. doi: 10.1016/j.ejpb.2018.07.010. Epub 2018 Jul 10. Eur J Pharm Biopharm. 2018. PMID: 30006244
-
A water-soluble, mucoadhesive quaternary ammonium chitosan-methyl-β-cyclodextrin conjugate forming inclusion complexes with dexamethasone.J Mater Sci Mater Med. 2018 Mar 30;29(4):42. doi: 10.1007/s10856-018-6048-2. J Mater Sci Mater Med. 2018. PMID: 29603020
-
Solid microparticles based on chitosan or methyl-β-cyclodextrin: a first formulative approach to increase the nose-to-brain transport of deferoxamine mesylate.J Control Release. 2015 Mar 10;201:68-77. doi: 10.1016/j.jconrel.2015.01.025. Epub 2015 Jan 22. J Control Release. 2015. PMID: 25620068 Free PMC article.
-
Synthesis, characterization and evaluation of thiolated quaternary ammonium-chitosan conjugates for enhanced intestinal drug permeation.Eur J Pharm Sci. 2009 Sep 10;38(2):112-20. doi: 10.1016/j.ejps.2009.06.006. Epub 2009 Jul 2. Eur J Pharm Sci. 2009. PMID: 19576984
-
2-Methyl-β-cyclodextrin grafted ammonium chitosan: synergistic effects of cyclodextrin host and polymer backbone in the interaction with amphiphilic prednisolone phosphate salt as revealed by NMR spectroscopy.Int J Pharm. 2020 Sep 25;587:119698. doi: 10.1016/j.ijpharm.2020.119698. Epub 2020 Jul 28. Int J Pharm. 2020. PMID: 32736017
Cited by
-
Antibacterial, Antibiofilm, and Antiadhesive Properties of Different Quaternized Chitosan Derivatives.Int J Mol Sci. 2019 Dec 13;20(24):6297. doi: 10.3390/ijms20246297. Int J Mol Sci. 2019. PMID: 31847119 Free PMC article.
-
Mass Spectrometry, Ion Mobility Separation and Molecular Modelling: A Powerful Combination for the Structural Characterisation of Substituted Cyclodextrins Mixtures.Int J Mol Sci. 2022 Nov 1;23(21):13352. doi: 10.3390/ijms232113352. Int J Mol Sci. 2022. PMID: 36362136 Free PMC article.
-
Thiolated 2-Methyl-β-Cyclodextrin as a Mucoadhesive Excipient for Poorly Soluble Drugs: Synthesis and Characterization.Polymers (Basel). 2022 Aug 3;14(15):3170. doi: 10.3390/polym14153170. Polymers (Basel). 2022. PMID: 35956685 Free PMC article.
-
Overview of processed excipients in ocular drug delivery: Opportunities so far and bottlenecks.Heliyon. 2023 Dec 23;10(1):e23810. doi: 10.1016/j.heliyon.2023.e23810. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38226207 Free PMC article. Review.
-
Nanoparticles Based on Quaternary Ammonium Chitosan-methyl-β-cyclodextrin Conjugate for the Neuropeptide Dalargin Delivery to the Central Nervous System: An In Vitro Study.Pharmaceutics. 2020 Dec 22;13(1):5. doi: 10.3390/pharmaceutics13010005. Pharmaceutics. 2020. PMID: 33374997 Free PMC article.
References
-
- Liu L, Zhu S. A study on the supramolecular structure of inclusion complex of β-cyclodextrin with prazosin hydrochloride. Carbohydr Polym. 2007;68(3):472–476.
-
- Marques HMC, Hadgraft J, Kellaway IW. Studies of cyclodextrin inclusion complexes I. The salbutamol–cyclodextrin complex as studied by phase solubility and DSC. Int J Pharm. 1990;63(3):259–266.
-
- Han HK, Shin HJ, Ha DH. Improved oral bioavailability of alendronate via mucoadhesive liposomal delivery system. Eur J Pharm Sci. 2012;46(5):500–507. - PubMed
-
- Ramalingam P, Ko YT. Improved oral delivery of resveratrol from N-trimethyl chitosan-g-palmitic acid surface-modified solid lipid nanoparticles. Colloids Surf B Biointerfaces. 2016;139:52–61. - PubMed
-
- Millotti G, Perera G, Vigl C, Pickl K, Sinner FM, Bernkop-Schnürch A. The use of chitosan-6-mercaptonicotinic acid nanoparticles for oral peptide drug delivery. Drug Deliv. 2011;18(3):190–197. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

