Liver-enriched activator protein 1 as an isoform of CCAAT/enhancer-binding protein beta suppresses stem cell features of hepatocellular carcinoma
- PMID: 29731667
- PMCID: PMC5927340
- DOI: 10.2147/CMAR.S160172
Liver-enriched activator protein 1 as an isoform of CCAAT/enhancer-binding protein beta suppresses stem cell features of hepatocellular carcinoma
Abstract
Purpose: Liver cancer stem cells (CSCs) are known to be associated with the development, survival, proliferation, metastasis, and recurrence of liver tumors. The aim of this study was to investigate the association of liver-enriched activator protein 1 (LAP1) with hepatocellular carcinoma (HCC) and liver CSCs (LCSCs) and explore the impact of LAP1 on LCSCs.
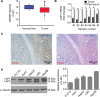
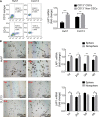
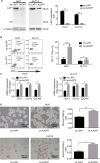
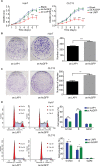
Materials and methods: Differences in LAP1 expression in liver cancer tissues versus matched para-tumoral liver tissues and LCSCs versus non-CSCs were analyzed by Western blotting, real-time polymerase chain reaction, immunohistochemistry, and flow cytometry. The effect of LAP1 on liver cancer cells was evaluated by the expression of CSC markers, oncosphere formation, proliferation, migration, and invasion in vitro. Cell cycle distribution and the number of apoptotic cells were analyzed to assess cell cycle and cell apoptosis. Furthermore, a mouse subcutaneous tumor implant model was established to explore the role of LAP1 in the development of HCC in vivo. Finally, the expression of CSC markers in paraffin-embedded sections was evaluated by immunofluorescence.
Results: LAP1 was weakly expressed in HCC tumors and cell lines and even weaker in LCSCs. LAP1 inhibited the expression of stem cell-associated genes and reduced the abilities of oncosphere formation, proliferation, migration, and invasion in vitro. Cell cycle assay revealed that LAP1 induced G1/G0 arrest. Furthermore, LAP1 decreased subcutaneous tumor-formation ability and the expression of CSC markers and Ki67 in vivo.
Conclusion: LAP1 suppressed the stem cell features of HCC, indicating that it possessed an antitumor effect in liver cancer, both in vitro and in vivo; therefore, LAP1 may prove to be a potential target in liver CSC-targeted therapy.
Keywords: HCC; LAP1; hepatocellular carcinoma; liver cancer stem cell; liver-enriched activator protein 1.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. - PubMed
-
- Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62(6):394–399. - PubMed
-
- Hao J, Zhang Y, Deng M, et al. MicroRNA control of epithelial-mesenchymal transition in cancer stem cells. Int J Cancer. 2014;135(5):1019–1027. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

