Genetic Ablation of EWS RNA Binding Protein 1 (EWSR1) Leads to Neuroanatomical Changes and Motor Dysfunction in Mice
- PMID: 29731676
- PMCID: PMC5934541
- DOI: 10.5607/en.2018.27.2.103
Genetic Ablation of EWS RNA Binding Protein 1 (EWSR1) Leads to Neuroanatomical Changes and Motor Dysfunction in Mice
Abstract
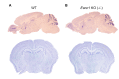
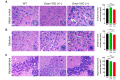
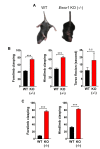
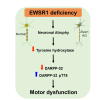
A recent study reveals that missense mutations of EWSR1 are associated with neurodegenerative disorders such as amyotrophic lateral sclerosis, but the function of wild-type (WT) EWSR1 in the central nervous system (CNS) is not known yet. Herein, we investigated the neuroanatomical and motor function changes in Ewsr1 knock out (KO) mice. First, we quantified neuronal nucleus size in the motor cortex, dorsal striatum and hippocampus of three different groups: WT, heterozygous Ewsr1 KO (+/-), and homozygous Ewsr1 KO (-/-) mice. The neuronal nucleus size was significantly smaller in the motor cortex and striatum of homozygous Ewsr1 KO (-/-) mice than that of WT. In addition, in the hippocampus, the neuronal nucleus size was significantly smaller in both heterozygous Ewsr1 KO (+/-) and homozygous Ewsr1 KO (-/-) mice. We then assessed motor function of Ewsr1 KO (-/-) and WT mice by a tail suspension test. Both forelimb and hindlimb movements were significantly increased in Ewsr1 KO (-/-) mice. Lastly, we performed immunohistochemistry to examine the expression of TH, DARPP-32, and phosphorylated (p)-DARPP-32 (Thr75) in the striatum and substantia nigra, which are associated with dopaminergic signaling. The immunoreactivity of TH and DARPP-32 was decreased in Ewsr1 KO (-/-) mice. Together, our results suggest that EWSR1 plays a significant role in neuronal morphology, dopaminergic signaling pathways, and motor function in the CNS of mice.
Keywords: DARPP-32; EWSR1; central nervous system (CNS); dopamine; motor function; neuron.
Figures

References
-
- Bertolotti A, Melot T, Acker J, Vigneron M, Delattre O, Tora L. EWS, but not EWS-FLI-1, is associated with both TFIID and RNA polymerase II: interactions between two members of the TET family, EWS and hTAFII68, and subunits of TFIID and RNA polymerase II complexes. Mol Cell Biol. 1998;18:1489–1497. - PMC - PubMed
-
- Yang L, Embree LJ, Tsai S, Hickstein DD. Oncoprotein TLS interacts with serine-arginine proteins involved in RNA splicing. J Biol Chem. 1998;273:27761–27764. - PubMed
-
- Chansky HA, Hu M, Hickstein DD, Yang L. Oncogenic TLS/ERG and EWS/Fli-1 fusion proteins inhibit RNA splicing mediated by YB-1 protein. Cancer Res. 2001;61:3586–3590. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

