Would You Use It With a Seal of Approval? Important Attributes of 2,4-Dinitrophenol (2,4-DNP) as a Hypothetical Pharmaceutical Product
- PMID: 29731723
- PMCID: PMC5919945
- DOI: 10.3389/fpsyt.2018.00124
Would You Use It With a Seal of Approval? Important Attributes of 2,4-Dinitrophenol (2,4-DNP) as a Hypothetical Pharmaceutical Product
Abstract
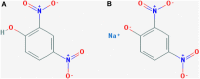
Background: 2,4-Dinitrophenol (2,4-DNP) is an effective but highly dangerous fat burner, not licensed for human consumption. Death cases reported for 2,4-DNP overdose, particularly among young adults, have raised concerns about the ineffective regulatory control, lack of education and risks associated with impurity, and the unknown concentration of 2,4-DNP purchased on the Internet.
Methods: Using a sequential mixed method design and based on a hypothetical scenario as if 2,4-DNP was a licensed pharmaceutical drug, first we conducted a qualitative study to explore what product attributes people consider when buying a weight-loss aid. Focus group interviews with six females and three males (mean age = 21.6 ± 1.8 years) were audiorecorded, transcribed verbatim, and subjected to thematic analysis. Sixteen attributes were identified for the Best-Worst Scale (BWS) in the quantitative survey with 106 participants (64% female, mean age = 27.1 ± 11.9 years), focusing on 2,4-DNP. Demographics, weight satisfaction, and risk for eating disorder data were collected.
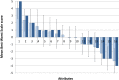
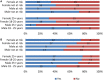
Results: In contrast to experienced users such as bodybuilders, our study participants approached 2,4-DNP cautiously. Attributes of 2,4-DNP as a hypothetical weight-loss drug comprised a range of desirable and avoidable features. Of the 16 selected attributes, BWS suggested that long-term side effects were the most and branding was the least important attribute. Effectiveness and short-term side effects were also essential. Those in the >25 year group showed least concerns for legality. Neutral BWS scores for cost, treatment, degree of lifestyle changes required, and specificity required for the hypothetical weight-loss drug to be effective were likely caused by disagreement about their importance among the participants, not indifference.
Conclusion: With advances in research, 2,4-DNP as a pharmaceutical drug in the future for treating neurodegenerative diseases and potentially for weight loss is not inconceivable. Caution is warranted for interpreting the BWS scores. Owing to the difference in what data represent at individual vs. population levels, with pooled data, the method correctly identifies attributes by which most people are satisfied but misrepresents attributes that are individually very important but not universally agreed. Whilst this may be an advantage in marketing applications, it limits the utility of BWS as a research tool.
Keywords: 2,4-dinitrophenol; DNP; bodybuilding; diet pill; eating disorder; fat burner; weight loss.
Figures



Similar articles
-
Russian roulette with unlicensed fat-burner drug 2,4-dinitrophenol (DNP): evidence from a multidisciplinary study of the internet, bodybuilding supplements and DNP users.Subst Abuse Treat Prev Policy. 2015 Oct 14;10:39. doi: 10.1186/s13011-015-0034-1. Subst Abuse Treat Prev Policy. 2015. PMID: 26466580 Free PMC article.
-
Being in control? A thematic content analysis of 14 in-depth interviews with 2,4-dinitrophenol users.Int J Drug Policy. 2018 Feb;52:106-114. doi: 10.1016/j.drugpo.2017.12.012. Epub 2018 Jan 11. Int J Drug Policy. 2018. PMID: 29331928
-
2,4 dinitrophenol: It's not just for men.Int J Drug Policy. 2021 Sep;95:102987. doi: 10.1016/j.drugpo.2020.102987. Epub 2020 Oct 16. Int J Drug Policy. 2021. PMID: 33077346
-
2,4 Dinitrophenol as Medicine.Cells. 2019 Mar 23;8(3):280. doi: 10.3390/cells8030280. Cells. 2019. PMID: 30909602 Free PMC article. Review.
-
Diet aid or aid to die: an update on 2,4-dinitrophenol (2,4-DNP) use as a weight-loss product.Arch Toxicol. 2020 Apr;94(4):1071-1083. doi: 10.1007/s00204-020-02675-9. Epub 2020 Feb 20. Arch Toxicol. 2020. PMID: 32078021 Review.
Cited by
-
Neuronal AMPK coordinates mitochondrial energy sensing and hypoxia resistance in C. elegans.FASEB J. 2020 Dec;34(12):16333-16347. doi: 10.1096/fj.202001150RR. Epub 2020 Oct 15. FASEB J. 2020. PMID: 33058299 Free PMC article.
-
Sublethal toxicities of 2,4-dinitrophenol as inferred from online self-reports.PLoS One. 2023 Sep 13;18(9):e0290630. doi: 10.1371/journal.pone.0290630. eCollection 2023. PLoS One. 2023. PMID: 37703241 Free PMC article.
-
Novel Strategies in the Early Detection and Treatment of Endothelial Cell-Specific Mitochondrial Dysfunction in Coronary Artery Disease.Antioxidants (Basel). 2023 Jun 28;12(7):1359. doi: 10.3390/antiox12071359. Antioxidants (Basel). 2023. PMID: 37507899 Free PMC article. Review.
-
Mitochondrial Uncoupling: A Fine-Tuning Knob for Mitochondria-Targeting Therapeutics for Coronary Artery Disease.J Atheroscler Thromb. 2022 Jun 1;29(6):811-813. doi: 10.5551/jat.ED185. Epub 2021 Sep 25. J Atheroscler Thromb. 2022. PMID: 34565766 Free PMC article. No abstract available.
-
One Does Not Fit All: European Study Shows Significant Differences in Value-Priorities in Clean Sport.Front Sports Act Living. 2021 May 24;3:662542. doi: 10.3389/fspor.2021.662542. eCollection 2021. Front Sports Act Living. 2021. PMID: 34109312 Free PMC article.
References
-
- Evans-Brown M, McVeigh J, Perkins C, Bellis MA. North West public health observatory. Human Enhancement Drugs: The Emerging Challenges to Public Health. Liverpool: Liverpool John Moores University; (2012).
LinkOut - more resources
Full Text Sources
Other Literature Sources

