Methotrexate remediates spinal cord injury in vivo and in vitro via suppression of endoplasmic reticulum stress-induced apoptosis
- PMID: 29731818
- PMCID: PMC5921236
- DOI: 10.3892/etm.2018.5973
Methotrexate remediates spinal cord injury in vivo and in vitro via suppression of endoplasmic reticulum stress-induced apoptosis
Abstract
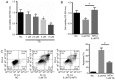
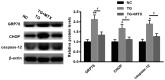
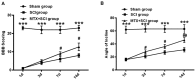
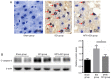
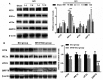
It has been suggested that endoplasmic reticulum stress (ERS) may induce apoptosis following spinal cord injury (SCI). Methotrexate (MTX) has been used as a long-term therapy regimen for rheumatoid arthritis. However, it is not clear whether MTX remediates SCI by inhibiting ERS. In the present study, to establish an in vitro ERS cell model, PC12 cells were pre-incubated with triglycerides (TG). MTT assays revealed that treatment with 1, 2.5, 5 and 10 µM TG decreased PC12 cell viability in a dose-dependent manner. Additionally, MTX treatment significantly reversed the TG-induced decrease in cell viability and increased apoptosis according to the flow cytometry assay (P<0.05). Notably, western blotting indicated that MTX significantly decreased levels of glucose-regulated protein (GRP)78, CCAAT-enhancer-binding protein homologous protein (CHOP) and caspase-12 expression (P<0.05), which were increased following treatment with TG. Furthermore, the in vivo role of MTX in a rat model of SCI was evaluated. The motor behavioral function of rats was improved following treatment with MTX according to Basso, Beattie and Bresnahan scoring (P<0.05). Terminal deoxynucleotidyl-transferase-mediated dUTP nick end staining indicated that there were no apoptotic cells present in sham rats. In the SCI model group, apoptotic cells were observed at day 7; however, the number of apoptotic cells was reduced following an additional 7 days of MTX administration. Furthermore, levels of ERS-associated proteins, including caspase-3, activating transcription factor 6, serine/threonine-protein kinase/endoribonuclease inositol-requiring enzyme 1 α, eukaryotic initiation factor 2 α and GRP78, were significantly increased following SCI; however, administration of MTX for 7 days significantly reversed this effect (P<0.05, P<0.01 and P<0.001). Therefore, MTX may improve SCI by suppressing ERS-induced apoptosis in vitro and in vivo.
Keywords: apoptosis; endoplasmic reticulum stress; methotrexate; spinal cord injury.
Figures
References
-
- Lee JY, Maeng S, Kang SR, Choi HY, Oh TH, Ju BG, Yune TY. Valproic acid protects motor neuron death by inhibiting oxidative stress and endoplasmic reticulum stress-mediated cytochrome C release after spinal cord injury. J Neurotrauma. 2014;31:582–594. doi: 10.1089/neu.2013.3146. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
