Correlation of Immunohistochemistry and Fluorescence in Situ Hybridization for HER-2 Assessment in Breast Cancer Patients: Single Centre Experience
- PMID: 29731922
- PMCID: PMC5927485
- DOI: 10.3889/oamjms.2018.124
Correlation of Immunohistochemistry and Fluorescence in Situ Hybridization for HER-2 Assessment in Breast Cancer Patients: Single Centre Experience
Abstract
Background: Accurate assessment of HER-2 is imperative in selecting patients for targeted therapy. Most commonly used test methods for HER-2 are immunohistochemistry (IHC) and fluorescence in situ hybridisation (FISH). We evaluated the concordance between FISH and IHC for HER-2 in breast cancer samples using Food and Drug Administration approved tests.
Material and methods: Archived paraffin tissue blocks from 73 breast cancer patients were used. HER-2 immunostaining was performed using Ventana anti-HER-2 monoclonal antibody. The FISH assay was performed using PathVysion™ HER-2 DNA Probe Kit.
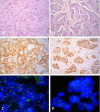
Results: Of the 73 cases 68.5% were IHC 0/1+, 15.07% were IHC 2+ and 16.44% were IHC 3+. Successful hybridisation was achieved in 72 cases. HER-2 FISH amplification was determined in 16.67% cases. Ten IHC 3+ and two IHC 2+ cases were FISH positive. Two of the IHC 3+ cases were FISH negative. Concordance rate was 100%, 18.18% and 83.33% for IHC 0/1+, 2+ and 3+ group, respectively. Total concordance was 84.72%, kappa 0.598 (p < 0.0001). The sensitivity of IHC in detecting IHC 2+ and IHC 3+ cases was 16.7% and 83.3%, and the specificity was 85% and 96.67%, respectively.
Conclusion: The consistency between the methods was highest for IHC negative and lowest for IHC equivocal cases. The immunohistochemistry showed high sensitivity for IHC 2+/3+ cases and high specificity for IHC 3+ cases. Our results support the view that false-positive rather than false-negative IHC results are a problem with HER-2/IHC testing, and that IHC should be used as an initial screening test, but IHC 2+/ 3+ results should be confirmed by FISH.
Keywords: Breast cancer; Fluorescence in situ hybridisation; HER –2; Immunohistochemistry.
Figures


Similar articles
-
Standardization and optimization of fluorescence in situ hybridization (FISH) for HER-2 assessment in breast cancer: A single center experience.Bosn J Basic Med Sci. 2018 May 20;18(2):132-140. doi: 10.17305/bjbms.2018.2519. Bosn J Basic Med Sci. 2018. PMID: 29389309 Free PMC article.
-
Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry.J Clin Oncol. 2000 Nov 1;18(21):3651-64. doi: 10.1200/JCO.2000.18.21.3651. J Clin Oncol. 2000. PMID: 11054438
-
Laboratory assessment of the status of Her-2/neu protein and oncogene in breast cancer specimens: comparison of immunohistochemistry assay with fluorescence in situ hybridisation assays.J Clin Pathol. 2000 May;53(5):374-81. doi: 10.1136/jcp.53.5.374. J Clin Pathol. 2000. PMID: 10889820 Free PMC article.
-
[HER-2 oncogene amplification assessment in invasive breast cancer by dual-color in situ hybridization (dc-CISH): a comparative study with fluorescent in situ hybridization (FISH)].Ann Pathol. 2011 Dec;31(6):472-9. doi: 10.1016/j.annpat.2011.10.013. Epub 2011 Nov 26. Ann Pathol. 2011. PMID: 22172120 Review. French.
-
HER-2 status and its clinicopathologic significance in breast cancer in patients from southwest China: re-evaluation of correlation between results from FISH and IHC.Int J Clin Exp Pathol. 2017 Jul 1;10(7):7270-7276. eCollection 2017. Int J Clin Exp Pathol. 2017. PMID: 31966566 Free PMC article. Review.
Cited by
-
Breast Carcinoma - A Comparative Study of Immunohistochemistry and Fluorescence in Situ Hybridization for Her-2 Assessment and Association of ER, PR, HER-2 and Ki-67 Expression with Clinico-Pathological Parameters.Iran J Pathol. 2022 Fall;17(4):435-442. doi: 10.30699/IJP.2022.538167.2712. Epub 2022 Sep 2. Iran J Pathol. 2022. PMID: 36532645 Free PMC article.
-
Clinicopathological Characteristics of Breast Cancer Patients with Equivocal Immunohistochemistry: A Prevalence-Based Statistical Analysis.Iran J Pathol. 2025 Summer;20(3):273-279. doi: 10.30699/ijp.2025.2045071.3378. Epub 2025 Jul 1. Iran J Pathol. 2025. PMID: 40746925 Free PMC article.
-
A review on methods for diagnosis of breast cancer cells and tissues.Cell Prolif. 2020 Jul;53(7):e12822. doi: 10.1111/cpr.12822. Epub 2020 Jun 12. Cell Prolif. 2020. PMID: 32530560 Free PMC article. Review.
References
-
- Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalised medicine. Oncologist. 2009;14(4):320–368. https://doi.org/10.1634/theoncologist.2008-0230. PMid:19346299. - PubMed
-
- Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Richard J, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131(1):18–43. PMid:19548375. - PubMed
-
- Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF. Guidelines for human epidermal growth factor receptor 2 testing: Biologic and methodologic considerations. J Clin Oncol. 2009;27(8):1323–1333. https://doi.org/10.1200/JCO.2007.14.8197. PMid:19204209. - PubMed
-
- Sapino A, Goia M, Recupero D, Marchiò C. Current challenges for HER2 testing in diagnostic pathology: State of the art and controversial issues. Front Oncol. 2013;3:129. https://doi.org/10.3389/fonc.2013.00129. PMid:23734345. PMCid: PMC3659312. - PMC - PubMed
-
- Lottner C, Schwarz S, Diermeier S, Hartmann A, Knuechel R, Hofstaedter F, et al. Simultaneous detection of HER2/neu gene amplification and protein overexpression in paraffin-embedded breast cancer. J Pathol. 2005;205(5):577–584. https://doi.org/10.1002/path.1742. PMid:15732132. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
