Correlation of Immunohistochemistry and Fluorescence in Situ Hybridization for HER-2 Assessment in Breast Cancer Patients: Single Centre Experience
- PMID: 29731922
- PMCID: PMC5927485
- DOI: 10.3889/oamjms.2018.124
Correlation of Immunohistochemistry and Fluorescence in Situ Hybridization for HER-2 Assessment in Breast Cancer Patients: Single Centre Experience
Abstract
Background: Accurate assessment of HER-2 is imperative in selecting patients for targeted therapy. Most commonly used test methods for HER-2 are immunohistochemistry (IHC) and fluorescence in situ hybridisation (FISH). We evaluated the concordance between FISH and IHC for HER-2 in breast cancer samples using Food and Drug Administration approved tests.
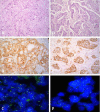
Material and methods: Archived paraffin tissue blocks from 73 breast cancer patients were used. HER-2 immunostaining was performed using Ventana anti-HER-2 monoclonal antibody. The FISH assay was performed using PathVysion™ HER-2 DNA Probe Kit.
Results: Of the 73 cases 68.5% were IHC 0/1+, 15.07% were IHC 2+ and 16.44% were IHC 3+. Successful hybridisation was achieved in 72 cases. HER-2 FISH amplification was determined in 16.67% cases. Ten IHC 3+ and two IHC 2+ cases were FISH positive. Two of the IHC 3+ cases were FISH negative. Concordance rate was 100%, 18.18% and 83.33% for IHC 0/1+, 2+ and 3+ group, respectively. Total concordance was 84.72%, kappa 0.598 (p < 0.0001). The sensitivity of IHC in detecting IHC 2+ and IHC 3+ cases was 16.7% and 83.3%, and the specificity was 85% and 96.67%, respectively.
Conclusion: The consistency between the methods was highest for IHC negative and lowest for IHC equivocal cases. The immunohistochemistry showed high sensitivity for IHC 2+/3+ cases and high specificity for IHC 3+ cases. Our results support the view that false-positive rather than false-negative IHC results are a problem with HER-2/IHC testing, and that IHC should be used as an initial screening test, but IHC 2+/ 3+ results should be confirmed by FISH.
Keywords: Breast cancer; Fluorescence in situ hybridisation; HER –2; Immunohistochemistry.
Figures


References
-
- Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalised medicine. Oncologist. 2009;14(4):320–368. https://doi.org/10.1634/theoncologist.2008-0230. PMid:19346299. - PubMed
-
- Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Richard J, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131(1):18–43. PMid:19548375. - PubMed
-
- Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF. Guidelines for human epidermal growth factor receptor 2 testing: Biologic and methodologic considerations. J Clin Oncol. 2009;27(8):1323–1333. https://doi.org/10.1200/JCO.2007.14.8197. PMid:19204209. - PubMed
-
- Sapino A, Goia M, Recupero D, Marchiò C. Current challenges for HER2 testing in diagnostic pathology: State of the art and controversial issues. Front Oncol. 2013;3:129. https://doi.org/10.3389/fonc.2013.00129. PMid:23734345. PMCid: PMC3659312. - PMC - PubMed
-
- Lottner C, Schwarz S, Diermeier S, Hartmann A, Knuechel R, Hofstaedter F, et al. Simultaneous detection of HER2/neu gene amplification and protein overexpression in paraffin-embedded breast cancer. J Pathol. 2005;205(5):577–584. https://doi.org/10.1002/path.1742. PMid:15732132. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
