Total body irradiation and iron chelation treatment are associated with pancreatic injury following pediatric hematopoietic stem cell transplantation
- PMID: 29731964
- PMCID: PMC5929407
- DOI: 10.18632/oncotarget.24646
Total body irradiation and iron chelation treatment are associated with pancreatic injury following pediatric hematopoietic stem cell transplantation
Abstract
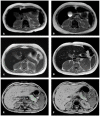
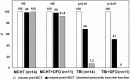
Whereas many studies have addressed the risk of organ dysfunction following hematopoietic stem cell transplantation (HSCT), little is known about pancreatic susceptibility in this setting. We aimed to investigate the effect of iron overload (IO) and total body irradiation (TBI) on pancreatic function of children undergoing HSCT. We retrospectively evaluated children admitted between 2012-2016 fulfilling the following criteria: normal pancreatic iron concentration (PIC), regular pancreatic function before HSCT, availability of abdominal magnetic resonance imaging with gradient-recalled-echo sequences and a full set of biochemical markers of IO and pancreatic function performed before HSCT and at discharge. We divided the patients according to the use of TBI or myeloablative chemotherapy (MCHT) in the conditioning regimen. All patients with severe IO or moderate IO with a high risk of engraftment delay or transplantation-related complications underwent chelation therapy with deferoxamine (DFO) from the first day of conditioning to discharge. 63 patients had a HSCT in the study period, 13 did not fulfill the inclusion criteria; 50 (25 in each group) are included in the analysis, and did not show differences at baseline evaluation. At follow up testing the TBI group showed a significantly higher PIC (107,8±100,3 μmol/g vs 28,4±37,9 in MCHT group, p<0,0001). In the TBI group the patients who had DFO treatment had higher PIC (223,2±48,8 μmol/g vs 55,7±10,5 without DFO treatment, p<0,0001), and all patients having PIC >100 μmol/g at follow up had DFO-based chelation therapy, versus 26% of those with lower PIC (p<0,0001). The number of patients presenting exocrine pancreatic dysfunctions one month after transplantation was significantly higher in the TBI group (48% vs 4%; p<0.0001). The mean pancreatic volume reduction was significantly greater in the TBI group (39,1% vs 0,9% in the MCHT group; p<0,05), and was significantly worse on those who received DFO therapy. Based on our data, we suggest that TBI is detrimental for pancreatic functions, and speculate that DFO may contribute to the rapid pancreatic IO observed in these patients.
Keywords: acute pancreatic iron overload; allogeneic HSCT; exocrine pancreatic dysfunction; pancreatic shrinkage; pediatric patients.
Conflict of interest statement
CONFLICTS OF INTEREST Natalia Maximova, Massimo Gregori, Roberto Simeone, Aurelio Sonzogni, Davide Zanon, Giulia Boz, Lorenzo D’Antiga declare that they have no conflict of interest.
Figures

References
-
- Majhail NS, Lazarus HM, Burns LJ. Iron overload in hematopoietic cell transplantatione. Bone Marrow Transplant. 2008;41:997–1003. - PubMed
-
- Altes A, Remacha AF, Sureda A, Martino R, Briones J, Canals C, Brunet S, Sierra J, Gimferrer E. Iron overload might increase transplant-related mortality in haematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:987–9. - PubMed
-
- Pullarkat V, Blanchard S, Tegtmeier B, Dagis A, Patane K, Ito J, Forman SJ. Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2008;42:799–805. - PubMed
-
- Kataoka K, Nannya Y, Hangaishi A, Imai Y, Chiba S, Takahashi T, Kurokawa M. Influence of pretransplantation serum ferritin on nonrelapse mortality after myeloablative and nonmyeloablative allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:195–204. - PubMed
-
- De Witte T. The role of iron in patients after bone marrow transplantation. Blood Reviews. 2008;22:S22–S28. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

