Intramolecular hydrogen-bonding in a cobalt aqua complex and electrochemical water oxidation activity
- PMID: 29732059
- PMCID: PMC5912104
- DOI: 10.1039/c7sc04960a
Intramolecular hydrogen-bonding in a cobalt aqua complex and electrochemical water oxidation activity
Abstract
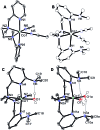
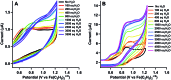
Water oxidation is catalysed in Nature by a redox cofactor embedded in a hydrogen-bonded network designed to orchestrate proton transfer throughout the challenging 4 electron reaction. In order to mimic aspects of this microenvironment, [CoLDMA(CH3CN)2][BF4]2 (2) was synthesized, where LDMA is a dipyridyldiamine ligand with two dimethylamine bases in the secondary coordination sphere. Structural characterization of the corresponding aqua complexes establish hydrogen bonding between the bound water and pendant base(s). Cyclic voltammetry of [CoLDMA(CH3CN)2][BF4]2 (2) reveals enhanced oxidative current upon titration with water and controlled potential electrolysis confirms evolution of O2. The related complex [CoLH(CH3CN)2][BF4]2 (1), which has the same primary coordination environment as 2 but lacks pendant bases, is inactive. The structural and electrochemical studies illustrate the role positioned proton relays can play in promoting redox reactivity.
Figures


Similar articles
-
Two-Electron-Induced Reorganization of Cobalt Coordination and Metal-Ligand Cooperative Redox Shifting Co(I) Reactivity toward CO2 Reduction.Inorg Chem. 2023 Feb 6;62(5):2326-2333. doi: 10.1021/acs.inorgchem.2c04071. Epub 2023 Jan 23. Inorg Chem. 2023. PMID: 36691700
-
A facile biomimetic catalytic activity through hydrogen atom abstraction by the secondary coordination sphere in manganese(III) complexes.Dalton Trans. 2020 Oct 20;49(40):14216-14230. doi: 10.1039/d0dt02431g. Dalton Trans. 2020. PMID: 33025999
-
The remarkable reactivity of high oxidation state ruthenium and osmium polypyridyl complexes.Inorg Chem. 2003 Dec 15;42(25):8140-60. doi: 10.1021/ic020731v. Inorg Chem. 2003. PMID: 14658865
-
A modular, energy-based approach to the development of nickel containing molecular electrocatalysts for hydrogen production and oxidation.Biochim Biophys Acta. 2013 Aug-Sep;1827(8-9):1123-39. doi: 10.1016/j.bbabio.2013.01.003. Epub 2013 Jan 11. Biochim Biophys Acta. 2013. PMID: 23313415 Review.
-
The impact of secondary coordination sphere engineering on water oxidation reactivity catalysed by molecular ruthenium complexes: a next-generation approach to develop advanced catalysts.Dalton Trans. 2022 Jul 12;51(27):10320-10337. doi: 10.1039/d2dt01124g. Dalton Trans. 2022. PMID: 35730327 Review.
Cited by
-
Low overpotential water oxidation at neutral pH catalyzed by a copper(ii) porphyrin.Chem Sci. 2019 Jan 7;10(9):2613-2622. doi: 10.1039/c8sc04529a. eCollection 2019 Mar 7. Chem Sci. 2019. PMID: 30996977 Free PMC article.
-
An intramolecular cobalt-peptoid complex as an efficient electrocatalyst for water oxidation at low overpotential.Chem Sci. 2024 Jul 17;15(32):12928-12938. doi: 10.1039/d4sc01182a. eCollection 2024 Aug 14. Chem Sci. 2024. PMID: 39148784 Free PMC article.
-
Early Metal Di(pyridyl) Pyrrolide Complexes with Second Coordination Sphere Arene-π Interactions: Ligand Binding and Ethylene Polymerization.ACS Omega. 2019 Sep 19;4(14):15879-15892. doi: 10.1021/acsomega.9b01788. eCollection 2019 Oct 1. ACS Omega. 2019. PMID: 31592458 Free PMC article.
-
A homogeneous cobalt complex mediated electro and photocatalytic O2/H2O interconversion in neutral water.iScience. 2023 Oct 12;26(11):108189. doi: 10.1016/j.isci.2023.108189. eCollection 2023 Nov 17. iScience. 2023. PMID: 37920669 Free PMC article.
-
Selective Cobalt-Mediated Formation of Hydrogen Peroxide from Water under Mild Conditions via Ligand Redox Non-Innocence.J Am Chem Soc. 2024 Mar 6;146(9):5855-5863. doi: 10.1021/jacs.3c11032. Epub 2024 Feb 20. J Am Chem Soc. 2024. PMID: 38375752 Free PMC article.
References
-
- Vogt L., Vinyard D. J., Khan S., Brudvig G. W. Curr. Opin. Chem. Biol. 2015;25:152–158. - PubMed
-
- McEvoy J. P., Brudvig G. W. Phys. Chem. Chem. Phys. 2004;6:4754–4763.
-
- Hwang H. J., Dilbeck P., Debus R. J., Burnap R. L. Biochemistry. 2007;46:11987–11997. - PubMed
-
- Meyer T. J., Huynh M. H. V., Thorp H. H. Angew. Chem., Int. Ed. 2007;46:5284–5304. - PubMed
-
- Barry B. A., Brahmachari U., Guo Z. Acc. Chem. Res. 2017;50(8):1937–1945. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

