Intramolecular hydride transfer onto arynes: redox-neutral and transition metal-free C(sp3)-H functionalization of amines
- PMID: 29732071
- PMCID: PMC5914459
- DOI: 10.1039/c8sc00181b
Intramolecular hydride transfer onto arynes: redox-neutral and transition metal-free C(sp3)-H functionalization of amines
Abstract
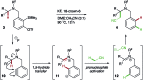
Transition metal-free intramolecular hydride transfer onto arynes is reported for the first time. This unique transformation is utilized in redox-neutral intermolecular α-functionalization reactions of different tertiary amines, generating C(sp3)-C(sp3/sp2/sp) bonds in a single synthetic operation. Deuterium labeling studies support initial cleavage of the α-C-H bond via intramolecular 1,5-hydride transfer onto the aryne, which leads to activation of a range of integrated pronucleophiles and ultimately affords a new approach to cross-dehydrogenative coupling reactions which utilize aryne intermediates.
Figures

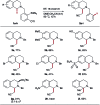
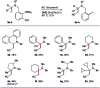
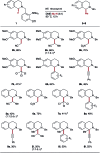
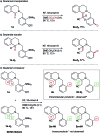
References
-
- Wencel-Delord J., Glorious F. Nat. Chem. 2013;5:369. - PubMed
-
-
Selected CDC reviews:
- Li C.-J. Acc. Chem. Res. 2009;42:335. - PubMed
- Zhang C., Tang C., Jiao N. Chem. Soc. Rev. 2012;41:3464. - PubMed
- Girard S. A., Knauber T., Li C.-J. Angew. Chem., Int. Ed. 2014;53:74. - PubMed
- Varun B. V., Dhineshkumar J., Bettadapur K. R., Siddaraju Y., Alagiri K., Prabhu K. R. Tetrahedron Lett. 2017;58:803.
-
LinkOut - more resources
Full Text Sources
Other Literature Sources

