Second-generation CK2α inhibitors targeting the αD pocket
- PMID: 29732088
- PMCID: PMC5916021
- DOI: 10.1039/c7sc05122k
Second-generation CK2α inhibitors targeting the αD pocket
Abstract
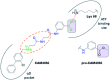
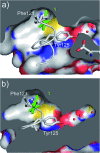
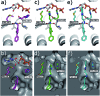
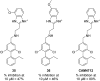
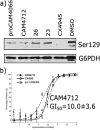
CK2 is a critical cell cycle regulator that also promotes various anti-apoptotic mechanisms. Development of ATP-non-competitive inhibitors of CK2 is a very attractive strategy considering that the ATP binding site is highly conserved among other kinases. We have previously utilised a pocket outside the active site to develop a novel CK2 inhibitor, CAM4066. Whilst CAM4066 bound to this new pocket it was also interacting with the ATP site: herein, we describe an example of a CK2α inhibitor that binds completely outside the active site. This second generation αD-site binding inhibitor, compound CAM4712 (IC50 = 7 μM, GI50 = 10.0 ± 3.6 μM), has numerous advantages over the previously reported CAM4066, including a reduction in the number of rotatable bonds, the absence of amide groups susceptible to the action of proteases and improved cellular permeability. Unlike with CAM4066, there was no need to facilitate cellular uptake by making a prodrug. Moreover, CAM4712 displayed no drop off between its ability to inhibit the kinase in vitro (IC50) and the ability to inhibit cell proliferation (GI50).
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

