Aligner-mediated cleavage of nucleic acids and its application to isothermal exponential amplification
- PMID: 29732089
- PMCID: PMC5916018
- DOI: 10.1039/c7sc05141g
Aligner-mediated cleavage of nucleic acids and its application to isothermal exponential amplification
Abstract
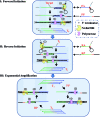
We herein describe a simple and versatile approach to use conventional nicking endonuclease (NEase) for programmable sequence-specific cleavage of DNA, termed aligner-mediated cleavage (AMC), and its application to DNA isothermal exponential amplification (AMC-based strand displacement amplification, AMC-SDA). AMC uses a hairpin-shaped DNA aligner (DA) that contains a recognition site in its stem and two side arms complementary to target DNA. Thus, it enables the loading of an NEase on DA's stem, localization to a specific locus through hybridization of the side arms with target DNA, and cleavage thereof. By using just one NEase, it is easy to make a break at any specific locus and tune the cleavage site to the single-nucleotide scale. This capability also endows the proposed AMC-SDA with excellent universality, since the cleavage of target DNA, followed by a polymerase-catalyzed extension along a particular primer as a key step for initiating SDA, no longer relies on any special sequence. Moreover, this manner of initiation facilitates the adoption of 3'-terminated primers, thus making AMC-SDA highly sensitive and highly specific, as well as simple primer design.
Figures





References
-
- Fleischhacker M., Schmidt B. Biochim. Biophys. Acta, Rev. Cancer. 2007;1775:181–232. - PubMed
-
- Chen X., Ba Y., Ma L., Cai X., Yin Y., Wang K., Guo J., Zhang Y., Chen J., Guo X., Li Q., Li X., Wang W., Zhang Y., Wang J., Jiang X., Xiang Y., Xu C., Zheng P., Zhang J., Li R., Zhang H., Shang X., Gong T., Ning G., Wang J., Zen K., Zhang J., Zhang C.-Y. Cell Res. 2008;18:997–1006. - PubMed
-
- Yáñez-Sedeño P., Agüí L., Villalonga R., Pingarrón J. M. Anal. Chim. Acta. 2014;823:1–19. - PubMed
-
- Zhao Y., Chen F., Li Q., Wang L., Fan C. Chem. Rev. 2015;115:12491–12545. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

