Increasing the bioactive space of peptide macrocycles by thioamide substitution
- PMID: 29732120
- PMCID: PMC5909342
- DOI: 10.1039/c7sc04671e
Increasing the bioactive space of peptide macrocycles by thioamide substitution
Abstract
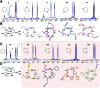
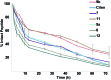
We show that substituting a single atom, O to S (amide to thioamide), in a peptide bond results in global restriction of the conformational flexibility in peptide macrocycles with minimal perturbation of the parent conformation. The van der Waals interactions between the C[double bond, length as m-dash]S group and the surrounding atoms are the major driving force in inducing the conformational restriction, resulting in well-defined structures of these cyclic peptides with static 3-D presentation of the pharmacophores. Utilizing this property of thioamides, we report the development of a superactive antagonist of pro-angiogenic αvβ3, αvβ5 and α5β1 integrins, which are responsible for cancer cell proliferation and survival. Using simple thio-scanning and spatial screening of a non-efficacious and conformationally flexible cyclic peptide, we could achieve a more than 105 fold enhancement in its efficacy in cellulo via a single O to S substitution. The developed peptide shows better efficacy in inhibiting the pro-angiogenic integrins than the drug candidate cilengitide, with a significantly enhanced serum half-life of 36 h compared to that of cilengitide (12 h). The long shelf-life, absence of non-specific toxicity and resistance to degradation of the thioamidated macrocyclic peptides in human serum suggest the promise of thioamides in markedly improving the affinity, efficacy and pharmacology of peptide macrocycles.
This journal is © The Royal Society of Chemistry 2018.
Figures

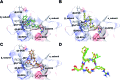

References
-
- Driggers E. M., Hale S. P., Lee J., Terrett N. K. Nat. Rev. Drug Discovery. 2008;7:608. - PubMed
-
- Hruby V. J. Life Sci. 1982;31:189. - PubMed
- Jackson S., Degrado W., Dwivedi A., Parthasarathy A., Higley A., Krywko J., Rockwell A., Markwalder J., Wells G., Wexler R., Mousa S., Harlow R. J. Am. Chem. Soc. 1994;116:3220.
- Hill T. A., Shepherd N. E., Diness F., Fairlie D. P. Angew. Chem., Int. Ed. 2014;53:13020. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

