Critical thresholds for intracranial pressure vary over time in non-craniectomised traumatic brain injury patients
- PMID: 29732476
- PMCID: PMC5996002
- DOI: 10.1007/s00701-018-3555-3
Critical thresholds for intracranial pressure vary over time in non-craniectomised traumatic brain injury patients
Abstract
Background: Intracranial pressure (ICP)- and cerebral perfusion pressure (CPP)-guided therapy is central to neurocritical care for traumatic brain injury (TBI) patients. We sought to identify time-dependent critical thresholds for mortality and unfavourable outcome for ICP and CPP in non-craniectomised TBI patients.
Methods: This is a retrospective cohort study of 355 patients with moderate-to-severe TBI who received ICP monitoring and were managed without decompressive craniectomy in a tertiary hospital neurocritical care unit. Patients were grouped in 2 × 2 tables according to survival/death or favourable/unfavourable outcomes at 6 months and serial thresholds of mean ICP and CPP, using increments of 0.1 and 0.5 mmHg respectively. Sequential chi-square analysis was performed, and the thresholds yielding the highest chi-square test statistic were taken as having the best discriminative value for outcome. This process was repeated over monitoring periods of 1, 3, 5 and 7 days and for each day of recording to establish time-dependent thresholds. The same analysis was performed for age and sex subgroups.
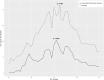
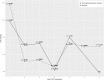
Results: Global ICP thresholds were 21.3 and 20.5 mmHg for mortality and unfavourable outcome respectively (p < 0.001). After the first day of ICP monitoring, ICP thresholds fell to between 15 and 20 mmHg and remained significant (p < 0.05). Significant time-dependent CPP thresholds for mortality or unfavourable outcome were often not identified, and no identifiable trends were produced.
Conclusion: Critical ICP thresholds in non-craniectomised TBI patients vary with time and fall below established ICP targets after the first day of monitoring.
Keywords: Cerebral perfusion pressure; Intracranial pressure; Neurocritical care; Neuromonitoring; Threshold; Traumatic brain injury.
Conflict of interest statement
Disclosures
FAZ has received salary support for dedicated research time, during which this project was partially completed. Such salary support came from the Cambridge Commonwealth Trust Scholarship, the Royal College of Surgeons of Canada-Harry S. Morton Travelling Fellowship in Surgery and the University of Manitoba Clinician Investigator Program.
DKM has consultancy agreements and/or research collaborations with GlaxoSmithKline Ltd.; Ornim Medical; Shire Medical Ltd.; Calico Inc.; Pfizer Ltd.; Pressura Ltd.; Glide Pharma Ltd.; and NeuroTraumaSciences LLC.
MC and PS have financial interest in a part of licencing fee for ICM+ software (Cambridge Enterprise Ltd., UK).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Informed consent
All data was fully anonymised, and no attempt was made to re-access clinical records for additional information. As such, formal patient or proxy consent was not required. Within our institution, patient data may be collected without consent, as long as it remains fully anonymised, with no method of tracing this back to an individual patient. Such requests for data curation in our TBI population have gone through our local Research Committee, as part of an NCCU protocol (Protocol #30), achieving approval for ongoing collection of such data, without the need for formal patient or proxy consent.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

