Tissue Engineering at the Blood-Contacting Surface: A Review of Challenges and Strategies in Vascular Graft Development
- PMID: 29732735
- PMCID: PMC6105365
- DOI: 10.1002/adhm.201701461
Tissue Engineering at the Blood-Contacting Surface: A Review of Challenges and Strategies in Vascular Graft Development
Abstract
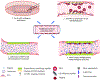
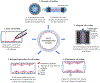
Tissue engineered vascular grafts (TEVGs) are beginning to achieve clinical success and hold promise as a source of grafting material when donor grafts are unsuitable or unavailable. Significant technological advances have generated small-diameter TEVGs that are mechanically stable and promote functional remodeling by regenerating host cells. However, developing a biocompatible blood-contacting surface remains a major challenge. The TEVG luminal surface must avoid negative inflammatory responses and thrombogenesis immediately upon implantation and promote endothelialization. The surface has therefore become a primary focus for research and development efforts. The current state of TEVGs is herein reviewed with an emphasis on the blood-contacting surface. General vascular physiology and developmental challenges and strategies are briefly described, followed by an overview of the materials currently employed in TEVGs. The use of biodegradable materials and stem cells requires careful control of graft composition, degradation behavior, and cell recruitment ability to ensure that a physiologically relevant vessel structure is ultimately achieved. The establishment of a stable monolayer of endothelial cells and the quiescence of smooth muscle cells are critical to the maintenance of patency. Several strategies to modify blood-contacting surfaces to resist thrombosis and control cellular recruitment are reviewed, including coatings of biomimetic peptides and heparin.
Keywords: blood-contacting surfaces; endothelialization; surface modification; vascular engineering; vascular grafts.
© 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
-
- Xu J, Murphy SL, Kochanek KD, Bastian BA, National Vital Statistics Reports 2016, 64, 39. - PubMed
-
- Mendis S, et al. , The World Health Organization, Geneva, Switzerland: 2014.
-
- Lin S, Sandig M, Mequanint K, Tissue engineering. Part A 2011, 17, 1561. - PubMed
-
- Rubanyi GM, J Cardiovasc Pharmacol 1993, 22 Suppl 4, S1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

