Weighing the Evidence of Efficacy of Oral PrEP for HIV Prevention in Women in Southern Africa
- PMID: 29732896
- PMCID: PMC6080090
- DOI: 10.1089/AID.2018.0031
Weighing the Evidence of Efficacy of Oral PrEP for HIV Prevention in Women in Southern Africa
Abstract
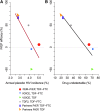
As oral tenofovir-based regimens for preexposure prophylaxis (PrEP) are adopted as standard of care for HIV prevention, their utilization in clinical trials among women in southern Africa will require an accurate estimate of oral PrEP efficacy in this population. This information is critical for women in choosing this prevention strategy, and in public health policy making. Estimates of the efficacy of oral PrEP regimens containing tenofovir have varied widely across trials that enrolled women, with some studies reporting high efficacy and others reporting no efficacy. Although poor adherence is strongly associated with lack of efficacy, other factors, such as mode of transmission (sexual vs. parenteral), predominant HIV subtype (C vs. non-C), intensity of exposure, and percentage of stable serodiscordant couples, may also contribute to the variation in efficacy estimates. In this article, we evaluate the evidence for PrEP efficacy in women and propose potential explanations for the observed differences in efficacy among studies. Our review emphasizes the need to continue to refine estimates of efficacy and effectiveness of tenofovir-based oral PrEP so as to best develop the next generation of HIV prevention tools, and to inform public policies directed toward HIV prevention.
Keywords: HIV prevention; adherence; oral PrEP efficacy; preexposure prophylaxis; southern African women.
Conflict of interest statement
Dr. Cohen is on the Advisory Board for both Merck and Gilead, and has served on Merck and Gilead advisory boards. The other authors declare no conflicts of interest.
Figures

References
-
- Lewden C, Chene G, Morlat P, et al. : HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr 2007;46:72–77 - PubMed
-
- Mocroft A, Ledergerber B, Katlama C, et al. : Decline in the AIDS and death rates in the EuroSIDA study: An observational study. Lancet 2003;362:22–29 - PubMed
-
- Palella FJ, Jr, Baker RK, Moorman AC, et al. : Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006;43:27–34 - PubMed
-
- Detels R, Munoz A, McFarlane G, et al. : Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA 1998;280:1497–1503 - PubMed
-
- Connor EM, Sperling RS, Gelber R, et al. : Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994;331:1173–1180 - PubMed
Appendix References
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. : Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012;367:423–34 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

