Prevalence of Rilpivirine and Etravirine Resistance Mutations in HIV-1 Subtype C-Infected Patients Failing Nevirapine or Efavirenz-Based Combination Antiretroviral Therapy in Botswana
- PMID: 29732907
- PMCID: PMC6079649
- DOI: 10.1089/AID.2017.0135
Prevalence of Rilpivirine and Etravirine Resistance Mutations in HIV-1 Subtype C-Infected Patients Failing Nevirapine or Efavirenz-Based Combination Antiretroviral Therapy in Botswana
Abstract
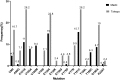
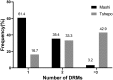
Rilpivirine (RPV) and Etravirine (ETR) are approved second-generation non-nucleoside reverse transcriptase inhibitors (NNRTIs) for HIV treatment. There is a cross-resistance HIV mutation profile between first- and second-generation NNRTI drugs. We determined the prevalence of HIV-1 drug resistance mutations (DRMs) to RPV and ETR in Botswana. A total of 168 HIV-1 polymerase gene sequences from participants failing nevirapine (NVP)- or efavirenz (EFV)-containing regimens were analyzed for DRMs using the Stanford University HIV drug resistance database. Forty-one sequences were from an adult antiretroviral therapy (ART) study, the Tshepo study, and 127 from a prevention of mother-to-child transmission (PMTCT) study, the Mashi study, all conducted in Botswana. Prevalence of RPV and ETR highest DRM in the adult ART study (n = 41) were K101E (26.2%), E138A (23.8%), and Y181C (26.2%). The PMTCT cohort's (n = 127) high prevalence mutations were Y181C (15.7%), E138A (15%), and K101E (11%). A total of 42.9% and 3.2% of patients in the adult ART study and PMTCT study, respectively, had three or more NNRTI mutations at virologic failure. We identified HIV-1 mutations conferring resistance to RPV and ETR even though they have not been used in Botswana. Of concern was the high proportion of sequences from the adult ART study that displayed multiple DRMs; as the number of NNRTI mutations increases, the level of cross-resistance increases. It is plausible that patients displaying such profiles maybe at increased risk of failing second-generation NNRTI drugs, hence, calls for genotyping in patients with prior NVP or efavirenz exposure before prescription of RPV- or ETR-containing cART.
Keywords: drug resistance; efavirenz; etravirine; nevirapine; rilpivirine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ministry of Health Botswana: Botswana National HIVand AIDS National Treatment Guidelines Ministry of Health, Gaborone, 2012
-
- Anta L, Llibre JM, Poveda E, et al. : Rilpivirine resistance mutations in HIV patients failing non-nucleoside reverse transcriptase inhibitor-based therapies. AIDS 2013;27:81–85 - PubMed
-
- FDA notifications. Rilpivirine approved for HIV treatment. AIDS Alert 2011;26:82–84 - PubMed
-
- FDA approves new HIV drug after priority review. U.S. Food and Drug Administration. AIDS Patient Care STDs 2008;22:159–161 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

