Blood Neutrophils Are Reprogrammed in Bronchiectasis
- PMID: 29733693
- PMCID: PMC6173062
- DOI: 10.1164/rccm.201712-2423OC
Blood Neutrophils Are Reprogrammed in Bronchiectasis
Abstract
Rationale: Excessive neutrophilic airway inflammation is the central feature of bronchiectasis, but little is known about neutrophils in bronchiectasis.
Objectives: To assess blood neutrophil phenotype in patients with bronchiectasis while stable and during exacerbations.
Methods: In the clinically stable arm of this study, there were eight healthy volunteers, eight patients with mild bronchiectasis, and eight patients with severe bronchiectasis. In addition, six patients with severe bronchiectasis were compared with six patients with community-acquired pneumonia at the start and end of an exacerbation. We assessed neutrophils for spontaneous apoptosis, cell surface marker expression, degranulation, reactive oxygen species generation, phagocytosis, and killing of Pseudomonas aeruginosa (PAO1). In addition, blood neutrophil function was compared with airway neutrophil function in bronchiectasis.
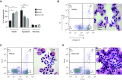
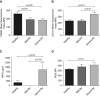
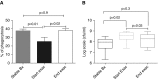
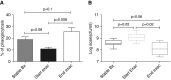
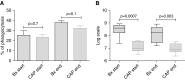
Measurements and main results: In stable bronchiectasis, compared with healthy volunteers, blood neutrophils had significantly prolonged viability, delayed apoptosis, increased CD62L shedding, upregulated CD11b expression, increased myeloperoxidase release, and impaired neutrophil phagocytosis and killing of PAO1. Bronchiectatic airway neutrophils had significantly lower bacterial phagocytosis and killing than their matched autologous blood neutrophils. Both blood and airway neutrophil phagocytosis and killing were impaired at the start of an exacerbation and improved following antibiotic treatment. In pneumonia, there was a significant improvement in phagocytosis and killing after treatment with antibiotics. During infections, there was no difference in phagocytosis, but there was significantly increased bacterial killing at the start and end of infection in pneumonia compared with bronchiectasis exacerbations.
Conclusions: In bronchiectasis stable state, peripheral blood neutrophils are reprogrammed and have prolonged survival. This impairs their functional ability of bacterial phagocytosis and killing, thereby perpetuating the vicious circle in bronchiectasis.
Keywords: bronchiectasis; infection; inflammation; neutrophils.
Figures



Comment in
-
One Small Step for Neutrophils, One Giant Leap for Bronchiectasis.Am J Respir Crit Care Med. 2018 Oct 1;198(7):828-830. doi: 10.1164/rccm.201804-0685ED. Am J Respir Crit Care Med. 2018. PMID: 29746143 No abstract available.
References
-
- Cole PJ. A new look at the pathogenesis, management of persistent bronchial sepsis: a 'vicious circle' hypothesis and its logical therapeutic connotations. Davies RJ. Strategies for the management of chronic bacterial sepsis. Oxford: Medicine Publishing Foundation; 1984. pp. 1–20.
-
- Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. - PubMed
-
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. - PubMed
-
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

