PepSAVI-MS reveals anticancer and antifungal cycloviolacins in Viola odorata
- PMID: 29734037
- PMCID: PMC6003877
- DOI: 10.1016/j.phytochem.2018.04.014
PepSAVI-MS reveals anticancer and antifungal cycloviolacins in Viola odorata
Abstract
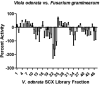
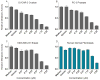
Widespread resistance to antimicrobial and cancer therapeutics is evolving in every country worldwide and has a direct impact on global health, agriculture and the economy. The specificity and selectivity of bioactive peptide natural products present a possible stopgap measure to address the ongoing deficit of new therapeutic compounds. PepSAVI-MS (Statistically-guided bioActive Peptides prioritized VIa Mass Spectrometry) is an adaptable method for the analysis of natural product libraries to rapidly identify bioactive peptides. This pipeline was validated via screening of the cyclotide-rich botanical species Viola odorata and identification of the known antimicrobial and anticancer cyclotide cycloviolacin O2. Herein we present and validate novel bioactivities of the anthelmintic V. odorata cyclotide, cycloviolacin O8 (cyO8), including micromolar anticancer activity against PC-3 prostate, MDA-MB-231 breast, and OVCAR-3 ovarian cancer cell lines and antifungal activity against the agricultural pathogen Fusarium graminearum. A reduction/alkylation strategy in tandem with PepSAVI-MS analysis also revealed several previously uncharacterized putatively bioactive cyclotides. Downstream implementation of ultraviolet photodissociation (UVPD) tandem mass spectrometry is demonstrated for cyO8 as a method to address traditionally difficult-to-sequence cyclotide species. This work emphasizes the therapeutic and agricultural potential of natural product bioactive peptides and the necessity of developing robust analytical tools to deconvolute nature's complexity.
Keywords: Antimicrobial peptides; Bioactive peptides; Cyclotides; Mass spectometry; Viola odorata, Violaceae.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures



References
-
- Ajesh K, Sreejith K. Peptide antibiotics: an alternative and effective antimicrobial strategy to circumvent fungal infections. Peptides. 2009;30:999–1006. - PubMed
-
- APVMA. PUBLIC RELEASE SUMMARY on the evaluation of the new active Clitoria ternatea in the product Sero-X Insecticide. Australian Pesticides and Veterinary Medicines Authority 2016
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

