Knockdown of CDK2AP1 in human embryonic stem cells reduces the threshold of differentiation
- PMID: 29734353
- PMCID: PMC5937771
- DOI: 10.1371/journal.pone.0196817
Knockdown of CDK2AP1 in human embryonic stem cells reduces the threshold of differentiation
Abstract
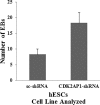
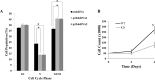
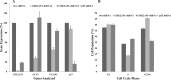
Recent studies have suggested a role for the Cyclin Dependent Kinase-2 Associated Protein 1 (CDK2AP1) in stem cell differentiation and self-renewal. In studies with mouse embryonic stem cells (mESCs) derived from generated mice embryos with targeted deletion of the Cdk2ap1 gene, CDK2AP1 was shown to be required for epigenetic silencing of Oct4 during differentiation, with deletion resulting in persistent self-renewal and reduced differentiation potential. Differentiation capacity was restored in these cells following the introduction of a non-phosphorylatible form of the retinoblastoma protein (pRb) or exogenous Cdk2ap1. In this study, we investigated the role of CDK2AP1 in human embryonic stem cells (hESCs). Using a shRNA to reduce its expression in hESCs, we found that CDK2AP1 knockdown resulted in a significant reduction in the expression of the pluripotency genes, OCT4 and NANOG. We also found that CDK2AP1 knockdown increased the number of embryoid bodies (EBs) formed when differentiation was induced. In addition, the generated EBs had significantly higher expression of markers of all three germ layers, indicating that CDK2AP1 knockdown enhanced differentiation. CDK2AP1 knockdown also resulted in reduced proliferation and reduced the percentage of cells in the S phase and increased cells in the G2/M phase of the cell cycle. Further investigation revealed that a higher level of p53 protein was present in the CDK2AP1 knockdown hESCs. In hESCs in which p53 and CDK2AP1 were simultaneously downregulated, OCT4 and NANOG expression was not affected and percentage of cells in the S phase of the cell cycle was not reduced. Taken together, our results indicate that the knockdown of CDK2AP1 in hESCs results in increased p53 and enhances differentiation and favors it over a self-renewal fate.
Conflict of interest statement
Figures


References
-
- Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. "Stemness": transcriptional profiling of embryonic and adult stem cells. Science. 2002;298(5593):597–600. doi: 10.1126/science.1072530 - DOI - PubMed
-
- Rao RR, Calhoun JD, Qin X, Rekaya R, Clark JK, Stice SL. Comparative transcriptional profiling of two human embryonic stem cell lines. Biotechnol Bioeng. 2004;88(3):273–86. doi: 10.1002/bit.20245 - DOI - PubMed
-
- Rao RR, Stice SL. Gene expression profiling of embryonic stem cells leads to greater understanding of pluripotency and early developmental events. Biol Reprod. 2004;71(6):1772–8. doi: 10.1095/biolreprod.104.030395 - DOI - PubMed
-
- Sharov AA, Piao Y, Matoba R, Dudekula DB, Qian Y, VanBuren V, et al. Transcriptome analysis of mouse stem cells and early embryos. PLoS Biol. 2003;1(3):E74 doi: 10.1371/journal.pbio.0000074 - DOI - PMC - PubMed
-
- Kim Y, McBride J, Kimlin L, Pae EK, Deshpande A, Wong DT. Targeted inactivation of p12, CDK2 associating protein 1, leads to early embryonic lethality. PLoS One. 2009;4(2):e4518 doi: 10.1371/journal.pone.0004518 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

