Pseudoprogression of brain tumors
- PMID: 29734497
- PMCID: PMC6175399
- DOI: 10.1002/jmri.26171
Pseudoprogression of brain tumors
Abstract
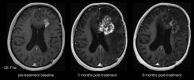
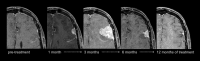
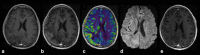
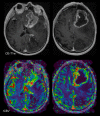
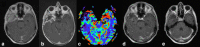
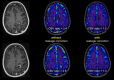
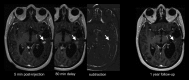
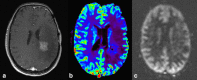
This review describes the definition, incidence, clinical implications, and magnetic resonance imaging (MRI) findings of pseudoprogression of brain tumors, in particular, but not limited to, high-grade glioma. Pseudoprogression is an important clinical problem after brain tumor treatment, interfering not only with day-to-day patient care but also the execution and interpretation of clinical trials. Radiologically, pseudoprogression is defined as a new or enlarging area(s) of contrast agent enhancement, in the absence of true tumor growth, which subsides or stabilizes without a change in therapy. The clinical definitions of pseudoprogression have been quite variable, which may explain some of the differences in reported incidences, which range from 9-30%. Conventional structural MRI is insufficient for distinguishing pseudoprogression from true progressive disease, and advanced imaging is needed to obtain higher levels of diagnostic certainty. Perfusion MRI is the most widely used imaging technique to diagnose pseudoprogression and has high reported diagnostic accuracy. Diagnostic performance of MR spectroscopy (MRS) appears to be somewhat higher, but MRS is less suitable for the routine and universal application in brain tumor follow-up. The combination of MRS and diffusion-weighted imaging and/or perfusion MRI seems to be particularly powerful, with diagnostic accuracy reaching up to or even greater than 90%. While diagnostic performance can be high with appropriate implementation and interpretation, even a combination of techniques, however, does not provide 100% accuracy. It should also be noted that most studies to date are small, heterogeneous, and retrospective in nature. Future improvements in diagnostic accuracy can be expected with harmonization of acquisition and postprocessing, quantitative MRI and computer-aided diagnostic technology, and meticulous evaluation with clinical and pathological data.
Level of evidence: 3 Technical Efficacy: Stage 2 J. Magn. Reson. Imaging 2018.
Keywords: MRI; brain neoplasms; diffusion; glioma; magnetic resonance imaging; perfusion weighted; proton magnetic resonance spectroscopy.
© 2018 The Authors Journal of Magnetic Resonance Imaging published by Wiley Periodicals, Inc. on behalf of International Society for Magnetic Resonance in Medicine.
Figures



References
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5‐year analysis of the EORTC‐NCIC trial. Lancet Oncol 2009;10:459–466. - PubMed
-
- de Wit MCY, de Bruin HG, Eijkenboom W, Sillevis Smitt PAE, van den Bent MJ. Immediate post‐radiotherapy changes in malignant glioma can mimic tumor progression. Neurology 2004;63:535–537. - PubMed
-
- Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol 2007;82:81–83. - PubMed
-
- Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys 2006;65:499–508. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

