A sucrose non-fermenting-1-related protein kinase-1 gene, IbSnRK1, improves starch content, composition, granule size, degree of crystallinity and gelatinization in transgenic sweet potato
- PMID: 29734529
- PMCID: PMC6330544
- DOI: 10.1111/pbi.12944
A sucrose non-fermenting-1-related protein kinase-1 gene, IbSnRK1, improves starch content, composition, granule size, degree of crystallinity and gelatinization in transgenic sweet potato
Abstract
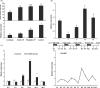
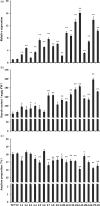
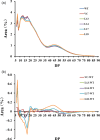
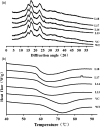
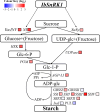
Sucrose non-fermenting-1-related protein kinase-1 (SnRK1) is an essential energy-sensing regulator and plays a key role in the global control of carbohydrate metabolism. The SnRK1 gene has been found to increase starch accumulation in several plant species. However, its roles in improving starch quality have not been reported to date. In this study, we found that the IbSnRK1 gene was highly expressed in the storage roots of sweet potato and strongly induced by exogenous sucrose. Its expression followed the circandian rhythm. Its overexpression not only increased starch content, but also decreased proportion of amylose, enlarged granule size and improved degree of crystallinity and gelatinization in transgenic sweet potato, which revealed, for the first time, the important roles of SnRK1 in improving starch quality of plants. The genes involved in starch biosynthesis pathway were systematically up-regulated, and the content of ADP-glucose as an important precursor for starch biosynthesis and the activities of key enzymes were significantly increased in transgenic sweet potato. These findings indicate that IbSnRK1 improves starch content and quality through systematical up-regulation of the genes and the increase in key enzyme activities involved in starch biosynthesis pathway in transgenic sweet potato. This gene has the potential to improve starch content and quality in sweet potato and other plants.
Keywords: IbSnRK1; starch content; starch quality; sweet potato.
© 2018 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Abe, N. , Nakamura, Y. and Fujita, N. (2013) Thermal properties, morphology of starch granules and crystallinity of endosperm starch in SSI and BE isozymes double mutant lines. J. Appl. glycosci. 60, 171–176.
-
- Ahn, Y.O. , Kim, S.H. , Kim, C.Y. , Lee, J.S. , Kwak, S.S. and Lee, H.S. (2010) Exogenous sucrose utilization and starch biosynthesis among sweetpotato cultivars. Carbohyd. Res. 345, 55–60. - PubMed
-
- Bahaji, A. , Li, J. , Sánchez‐López, Á.M. , Baroja‐Fernández, E. , Muñoz, F.J. , Ovecka, M. , Almagro, G. et al. (2014) Starch biosynthesis, its regulation and biotechnological approaches to improve crop yields. Biotechnol. Adv. 32, 87–106. - PubMed
-
- Ball, S.G. and Morell, M.K. (2003) From bacterial glycogen to starch: understanding the biogenesis of the plant starch granule. Annu. Rev. Plant Biol. 54, 207–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

