Thermosensitive Injectable Hydrogel for Simultaneous Intraperitoneal Delivery of Doxorubicin and Prevention of Peritoneal Adhesion
- PMID: 29734717
- PMCID: PMC5983626
- DOI: 10.3390/ijms19051373
Thermosensitive Injectable Hydrogel for Simultaneous Intraperitoneal Delivery of Doxorubicin and Prevention of Peritoneal Adhesion
Abstract
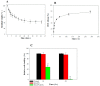
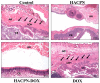
To improve intraperitoneal chemotherapy and to prevent postsurgical peritoneal adhesion, we aimed to develop a drug delivery strategy for controlled release of a chemotherapeutic drug from the intraperitoneally injected thermosensitive poly(N-isopropylacrylamide)-based hydrogel (HACPN), which is also endowed with peritoneal anti-adhesion properties. Anticancer drug doxorubicin (DOX) was loaded into the hydrogel (HACPN-DOX) to investigate the chemotherapeutic and adhesion barrier effects in vivo. A burst release followed by sustained release of DOX from HACPN-DOX was found due to gradual degradation of the hydrogel. Cell culture studies demonstrated the cytotoxicity of released DOX toward CT-26 mouse colon carcinoma cells in vitro. Using peritoneal carcinomatosis animal model in BALB/c mice with intraperitoneally injected CT-26 cells, animals treated with HACPN-DOX revealed the best antitumor efficacy judging from tumor weight and volume, survival rate, and bioluminescence signal intensity when compared with treatment with free DOX at the same drug dosage. HACPN (or HACPN-DOX) also significantly reduced the risk of postoperative peritoneal adhesion, which was generated by sidewall defect-cecum abrasion in tumor-bearing BALB/c mice, from gross and histology analyses. This study could create a paradigm to combine controlled drug release with barrier function in a single drug-loaded injectable hydrogel to enhance the intraperitoneal chemotherapeutic efficacy while simultaneously preventing postsurgical adhesion.
Keywords: anti-adhesion; anticancer; chemotherapy; doxorubicin; injectable hydrogel; thermosensitive.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Armstrong D., Bundy B., Wenzel L., Ozols R., Bundy B., Greer B. Intraperitoneal chemotherapy for ovarian cancer. N. Eng. J. Med. 2006;2006:1641–1643. - PubMed
-
- Elias D., Blot F., El Otmany A., Antoun S., Lasser P., Boige V., Rougier P., Ducreux M. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92:71–76. doi: 10.1002/1097-0142(20010701)92:1<71::AID-CNCR1293>3.0.CO;2-9. - DOI - PubMed
-
- Esquivel J., Sticca R., Sugarbaker P., Levine E., Yan T., Alexander R., Baratti D., Bartlett D., Barone R., Barrios P. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: A consensus statement. Ann. Surg. Oncol. 2007;14:128–133. doi: 10.1245/s10434-006-9185-7. - DOI - PubMed
-
- Verwaal V.J., Bruin S., Boot H., van Slooten G., van Tinteren H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 2008;15:2426–2432. doi: 10.1245/s10434-008-9966-2. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

