Adverse Drug Reactions (ADR) and Emergencies
- PMID: 29735005
- PMCID: PMC5949373
- DOI: 10.3238/arztebl.2018.0251
Adverse Drug Reactions (ADR) and Emergencies
Abstract
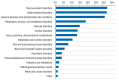
Background: Adverse drug reactions (ADR) are a common reason for emergency room visits and for hospitalization. An ADR is said to have occurred when the patient's symptoms and signs are considered to be possibly, probably, or definitely related to the intake of a drug.
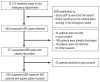
Methods: In four large hospital emergency departments, one in each of four German cities ( Ulm, Fürth, Bonn, and Stuttgart), the percentage of suspected ADR cases among all patients presenting to the emergency room was determined during a 30-day period of observation. ADRs were ascertained by screening the digital records of all patients seen in the emergency room; causality was assessed as specified by the WHO-UMC (Uppsala Monitoring Center).
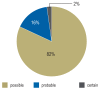
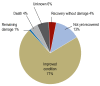
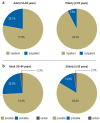
Results: ADR were sought in a total of 10 174 emergency department visits. 665 cases of suspected ADR were found, yielding a prevalence of 6.5%. The prevalence of ADR among patients with documented drug intake was 11.6%. Among the patients with documented suspected ADRs, 89% were hospitalized (in contrast to the 43.7% hospitalization rate in the entire group of 10 174 emergency department visits). A possible causal relationship between the patient's symptoms and signs and the intake of a drug was found in 74-84% of cases. Patients with ADR were found to be taking a median of 7 different drugs simultaneously.
Conclusion: Adverse drug reactions are a relevant cause of emergency department visits, accounting for 6.5% of the total visits in this study, and often lead to hospital admission. The ADRED (Adverse Drug Reactions in Emergency Departments) study, which is now being conducted, is intended to shed further light on their causes, patient risk factors, and potential avoidability.
Figures



Comment in
-
Increase in Risk of Acute Confusional State in Dementia Patients.Dtsch Arztebl Int. 2018 Sep 3;115(35-36):594. doi: 10.3238/arztebl.2018.0594a. Dtsch Arztebl Int. 2018. PMID: 30236219 Free PMC article. No abstract available.
References
-
- Bundesministerium für Gesundheit, Aktionsplan 2016-2019 zur Verbesserung der Arzneimitteltherapiesicherheit in Deutschland. https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Download...(last accessed on 27. June 2017)
-
- Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006;296:1858–1866. - PubMed
-
- van der Hooft CS, Dieleman JP, Siemes C, et al. Adverse drug reaction-related hospitalisations: a population-based cohort study. Pharmacoepidemiol Drug Saf. 2008;17:365–371. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

