A Capsular Polysaccharide-Specific Antibody Alters Streptococcus pneumoniae Gene Expression during Nasopharyngeal Colonization of Mice
- PMID: 29735523
- PMCID: PMC6013671
- DOI: 10.1128/IAI.00300-18
A Capsular Polysaccharide-Specific Antibody Alters Streptococcus pneumoniae Gene Expression during Nasopharyngeal Colonization of Mice
Abstract
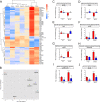
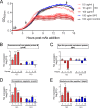
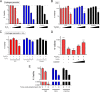
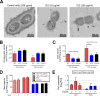
Pneumococcal conjugate vaccines (PCV) elicit opsonophagocytic (opsonic) antibodies to pneumococcal capsular polysaccharides (PPS) and reduce nasopharyngeal (NP) colonization by vaccine-included Streptococcus pneumoniae serotypes. However, nonopsonic antibodies may also be important for protection against pneumococcal disease. For example, 1E2, a mouse IgG1 monoclonal antibody (MAb) to the serotype 3 (ST3) PPS (PPS3), reduced ST3 NP colonization in mice and altered ST3 gene expression in vitro Here, we determined whether 1E2 affects ST3 gene expression in vivo during colonization of mice by performing RNA sequencing on NP lavage fluid from ST3-infected mice treated with 1E2, a control MAb, or phosphate-buffered saline. Compared to the results for the controls, 1E2 significantly altered the expression of over 50 genes. It increased the expression of the piuBCDA operon, which encodes an iron uptake system, and decreased the expression of dpr, which encodes a protein critical for resistance to oxidative stress. 1E2-mediated effects on ST3 in vivo required divalent binding, as Fab fragments did not reduce NP colonization or alter ST3 gene expression. In vitro, 1E2 induced dose-dependent ST3 growth arrest and altered piuB and dpr expression, whereas an opsonic PPS3 MAb, 5F6, did not. 1E2-treated bacteria were more sensitive to hydrogen peroxide and the iron-requiring antibiotic streptonigrin, suggesting that 1E2 may increase iron import and enhance sensitivity to oxidative stress. Finally, 1E2 also induced rapid capsule shedding in vitro, suggesting that this may initiate 1E2-induced changes in sensitivity to oxidative stress and gene expression. Our data reveal a novel mechanism of direct, antibody-mediated antibacterial activity that could inform new directions in antipneumococcal therapy and vaccine development.
Keywords: Streptococcus pneumoniae; antibody function; capsule; iron acquisition; monoclonal antibodies; nasopharyngeal colonization; pneumococcus; serotype 3.
Copyright © 2018 American Society for Microbiology.
Figures
Similar articles
-
Isolation and Characterization of Human Monoclonal Antibodies to Pneumococcal Capsular Polysaccharide 3.Microbiol Spectr. 2021 Dec 22;9(3):e0144621. doi: 10.1128/Spectrum.01446-21. Epub 2021 Nov 10. Microbiol Spectr. 2021. PMID: 34756090 Free PMC article.
-
Reduction of Streptococcus pneumoniae Colonization and Dissemination by a Nonopsonic Capsular Polysaccharide Antibody.mBio. 2016 Feb 2;7(1):e02260-15. doi: 10.1128/mBio.02260-15. mBio. 2016. PMID: 26838726 Free PMC article.
-
A serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibody requires Fcγ receptor III and macrophages to mediate protection against pneumococcal pneumonia in mice.Infect Immun. 2012 Apr;80(4):1314-22. doi: 10.1128/IAI.06081-11. Epub 2012 Jan 30. Infect Immun. 2012. PMID: 22290146 Free PMC article.
-
The host immune dynamics of pneumococcal colonization: implications for novel vaccine development.Hum Vaccin Immunother. 2014;10(12):3688-99. doi: 10.4161/21645515.2014.979631. Hum Vaccin Immunother. 2014. PMID: 25668673 Free PMC article. Review.
-
Streptococcus pneumoniae Capsular Polysaccharide.Microbiol Spectr. 2019 Mar;7(2):10.1128/microbiolspec.gpp3-0019-2018. doi: 10.1128/microbiolspec.GPP3-0019-2018. Microbiol Spectr. 2019. PMID: 30977464 Free PMC article. Review.
Cited by
-
Effects of human immunoglobulin A on Cryptococcus neoformans morphology and gene expression.Microbiol Spectr. 2025 Apr;13(4):e0200824. doi: 10.1128/spectrum.02008-24. Epub 2025 Feb 21. Microbiol Spectr. 2025. PMID: 39982066 Free PMC article.
-
Patient-Derived Antibody Data Yields Development of Broadly Cross-Protective Monoclonal Antibody against ST258 Carbapenem-Resistant Klebsiella pneumoniae.Microbiol Spectr. 2022 Aug 31;10(4):e0176022. doi: 10.1128/spectrum.01760-22. Epub 2022 Jul 11. Microbiol Spectr. 2022. PMID: 35862974 Free PMC article.
-
The Role of IgG Subclass in Antibody-Mediated Protection against Carbapenem-Resistant Klebsiella pneumoniae.mBio. 2020 Sep 8;11(5):e02059-20. doi: 10.1128/mBio.02059-20. mBio. 2020. PMID: 32900809 Free PMC article.
-
Synergistic Protection against Secondary Pneumococcal Infection by Human Monoclonal Antibodies Targeting Distinct Epitopes.J Immunol. 2023 Jan 1;210(1):50-60. doi: 10.4049/jimmunol.2200349. Epub 2022 Nov 9. J Immunol. 2023. PMID: 36351696 Free PMC article.
-
Human IgM Inhibits the Formation of Titan-Like Cells in Cryptococcus neoformans.Infect Immun. 2020 Mar 23;88(4):e00046-20. doi: 10.1128/IAI.00046-20. Print 2020 Mar 23. Infect Immun. 2020. PMID: 31988178 Free PMC article.
References
-
- Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, Slack M, Ladhani SN, Miller E, Goldblatt D. 2014. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis 14:839–846. doi: 10.1016/S1473-3099(14)70822-9. - DOI - PubMed
-
- Juergens C, Patterson S, Trammel J, Greenberg D, Givon-Lavi N, Cooper D, Gurtman A, Gruber WC, Scott DA, Dagan R. 2014. Post hoc analysis of a randomized double-blind trial of the correlation of functional and binding antibody responses elicited by 13-valent and 7-valent pneumococcal conjugate vaccines and association with nasopharyngeal colonization. Clin Vaccine Immunol 21:1277–1281. doi: 10.1128/CVI.00172-14. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

