Population Pharmacokinetic Model and Meta-analysis of Outcomes of Amphotericin B Deoxycholate Use in Adults with Cryptococcal Meningitis
- PMID: 29735567
- PMCID: PMC6021666
- DOI: 10.1128/AAC.02526-17
Population Pharmacokinetic Model and Meta-analysis of Outcomes of Amphotericin B Deoxycholate Use in Adults with Cryptococcal Meningitis
Erratum in
-
Correction for Stott et al., "Population Pharmacokinetic Model and Meta-analysis of Outcomes of Amphotericin B Deoxycholate Use in Adults with Cryptococcal Meningitis".Antimicrob Agents Chemother. 2018 Nov 26;62(12):e02249-18. doi: 10.1128/AAC.02249-18. Print 2018 Dec. Antimicrob Agents Chemother. 2018. PMID: 30478182 Free PMC article. No abstract available.
Abstract
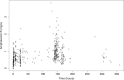
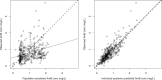
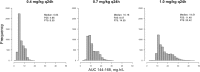
There is a limited understanding of the population pharmacokinetics (PK) and pharmacodynamics (PD) of amphotericin B deoxycholate (DAmB) for cryptococcal meningitis. A PK study was conducted in n = 42 patients receiving DAmB (1 mg/kg of body weight every 24 h [q24h]). A 2-compartment PK model was developed. Patient weight influenced clearance and volume in the final structural model. Monte Carlo simulations estimated drug exposure associated with various DAmB dosages. A search was conducted for trials reporting outcomes of treatment of cryptococcal meningitis patients with DAmB monotherapy, and a meta-analysis was performed. The PK parameter means (standard deviations) were as follows: clearance, 0.03 (0.01) × weight + 0.67 (0.01) liters/h; volume, 0.82 (0.80) × weight + 1.76 (1.29) liters; first-order rate constant from central compartment to peripheral compartment, 5.36 (6.67) h-1; first-order rate constant from peripheral compartment to central compartment, 9.92 (12.27) h-1 The meta-analysis suggested that the DAmB dosage explained most of the heterogeneity in cerebrospinal fluid (CSF) sterility outcomes but not in mortality outcomes. Simulations of values corresponding to the area under concentration-time curve from h 144 to h 168 (AUC144-168) resulted in median (interquartile range) values of 5.83 mg · h/liter (4.66 to 8.55), 10.16 mg · h/liter (8.07 to 14.55), and 14.51 mg · h/liter (11.48 to 20.42) with dosages of 0.4, 0.7, and 1.0 mg/kg q24h, respectively. DAmB PK is described adequately by a linear model that incorporates weight with clearance and volume. Interpatient PK variability is modest and unlikely to be responsible for variability in clinical outcomes. There is discordance between the impact that drug exposure has on CSF sterility and its impact on mortality outcomes, which may be due to cerebral pathology not reflected in CSF fungal burden, in addition to clinical variables.
Keywords: amphotericin B deoxycholate; cryptococcal meningitis; meta-analysis; pharmacodynamics; pharmacokinetics; population pharmacokinetics.
Copyright © 2018 Stott et al.
Figures



References
-
- Jarvis JN, Meintjes G, Rebe K, Williams GN, Bicanic T, Williams A, Schutz C, Bekker LG, Wood R, Harrison TS. 2012. Adjunctive interferon-gamma immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS 26:1105–1113. doi:10.1097/QAD.0b013e3283536a93. - DOI - PMC - PubMed
-
- Jarvis JN, Bicanic T, Loyse A, Namarika D, Jackson A, Nussbaum JC, Longley N, Muzoora C, Phulusa J, Taseera K, Kanyembe C, Wilson D, Hosseinipour MC, Brouwer AE, Limmathurotsakul D, White N, van der Horst C, Wood R, Meintjes G, Bradley J, Jaffar S, Harrison T. 2014. Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 58:736–745. doi:10.1093/cid/cit794. - DOI - PMC - PubMed
-
- Day JN, Chau TT, Wolbers M, Mai PP, Dung NT, Mai NH, Phu NH, Nghia HD, Phong ND, Thai CQ, Thai le H, Chuong LV, Sinh DX, Duong VA, Hoang TN, Diep PT, Campbell JI, Sieu TP, Baker SG, Chau NV, Hien TT, Lalloo DG, Farrar JJ. 2013. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med 368:1291–1302. doi:10.1056/NEJMoa1110404. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

