High-Dose Versus Low-Dose Pitavastatin in Japanese Patients With Stable Coronary Artery Disease (REAL-CAD): A Randomized Superiority Trial
- PMID: 29735587
- PMCID: PMC5959207
- DOI: 10.1161/CIRCULATIONAHA.117.032615
High-Dose Versus Low-Dose Pitavastatin in Japanese Patients With Stable Coronary Artery Disease (REAL-CAD): A Randomized Superiority Trial
Erratum in
-
Correction to: High-Dose Versus Low-Dose Pitavastatin in Japanese Patients With Stable Coronary Artery Disease (REAL-CAD).Circulation. 2019 Apr 2;139(14):e836. doi: 10.1161/CIR.0000000000000676. Circulation. 2019. PMID: 30933616 Free PMC article. No abstract available.
Abstract
Background: Current guidelines call for high-intensity statin therapy in patients with cardiovascular disease on the basis of several previous "more versus less statins" trials. However, no clear evidence for more versus less statins has been established in an Asian population.
Methods: In this prospective, multicenter, randomized, open-label, blinded end point study, 13 054 Japanese patients with stable coronary artery disease who achieved low-density lipoprotein cholesterol (LDL-C) <120 mg/dL during a run-in period (pitavastatin 1 mg/d) were randomized in a 1-to-1 fashion to high-dose (pitavastatin 4 mg/d; n=6526) or low-dose (pitavastatin 1 mg/d; n=6528) statin therapy. The primary end point was a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, or unstable angina requiring emergency hospitalization. The secondary composite end point was a composite of the primary end point and clinically indicated coronary revascularization excluding target-lesion revascularization at sites of prior percutaneous coronary intervention.
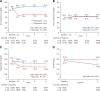
Results: The mean age of the study population was 68 years, and 83% were male. The mean LDL-C level before enrollment was 93 mg/dL with 91% of patients taking statins. The baseline LDL-C level after the run-in period on pitavastatin 1 mg/d was 87.7 and 88.1 mg/dL in the high-dose and low-dose groups, respectively. During the entire course of follow-up, LDL-C in the high-dose group was lower by 14.7 mg/dL than in the low-dose group (P<0.001). With a median follow-up of 3.9 years, high-dose as compared with low-dose pitavastatin significantly reduced the risk of the primary end point (266 patients [4.3%] and 334 patients [5.4%]; hazard ratio, 0.81; 95% confidence interval, 0.69-0.95; P=0.01) and the risk of the secondary composite end point (489 patients [7.9%] and 600 patients [9.7%]; hazard ratio, 0.83; 95% confidence interval, 0.73-0.93; P=0.002). High-dose pitavastatin also significantly reduced the risks of several other secondary end points such as all-cause death, myocardial infarction, and clinically indicated coronary revascularization. The results for the primary and the secondary composite end points were consistent across several prespecified subgroups, including the low (<95 mg/dL) baseline LDL-C subgroup. Serious adverse event rates were low in both groups.
Conclusions: High-dose (4 mg/d) compared with low-dose (1 mg/d) pitavastatin therapy significantly reduced cardiovascular events in Japanese patients with stable coronary artery disease.
Clinical trial registration: URL: https://www.clinicaltrials.gov. Unique identifier: NCT01042730.
Keywords: cholesterol, LDL; coronary artery disease; hydroxymethylglutaryl-CoA reductase inhibitors; long-term adverse effects; secondary prevention; stroke.
© 2018 The Authors.
Figures


Comment in
-
Extending the "Lower is Better" Principle to Japanese and Possibly Other Asian Populations.Circulation. 2018 May 8;137(19):2010-2012. doi: 10.1161/CIRCULATIONAHA.117.033001. Circulation. 2018. PMID: 29735588 No abstract available.
-
High- Versus Low-Dose Statin: Effects on Cardiovascular Events and All-Cause Death.Circulation. 2018 May 8;137(19):2013-2015. doi: 10.1161/CIRCULATIONAHA.118.034407. Circulation. 2018. PMID: 29735589 No abstract available.
-
Letter by Gong et al Regarding Article, "High-Dose Versus Low-Dose Pitavastatin in Japanese Patients With Stable Coronary Artery Disease (REAL-CAD): A Randomized Superiority Trial".Circulation. 2018 Dec 4;138(23):2726-2727. doi: 10.1161/CIRCULATIONAHA.118.036001. Circulation. 2018. PMID: 30571268 No abstract available.
-
Response by Kimura et al to Letters Regarding Article, "High-Dose Versus Low-Dose Pitavastatin in Japanese Patients With Stable Coronary Artery Disease (REAL-CAD): A Randomized Superiority Trial".Circulation. 2018 Dec 4;138(23):2728-2729. doi: 10.1161/CIRCULATIONAHA.118.036944. Circulation. 2018. PMID: 30571270 No abstract available.
-
Letter by Ye et al Regarding Article, "High-Dose Versus Low-Dose Pitavastatin in Japanese Patients With Stable Coronary Artery Disease (REAL-CAD): A Randomized Superiority Trial".Circulation. 2018 Dec 4;138(23):2724-2725. doi: 10.1161/CIRCULATIONAHA.118.035849. Circulation. 2018. PMID: 30571271 No abstract available.
References
-
- Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.CIR.97.18.1837. - PubMed
-
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383, 1389. doi: 10.1016/S0140-6736(94)90566-5. - PubMed
-
- Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels: Cholesterol and Recurrent Events Trial Investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. - PubMed
-
- West of Scotland Coronary Prevention Study Group. Influence of pravastatin and plasma lipids on clinical events in the West of Scotland Coronary Prevention Study (WOSCOPS). Circulation. 1998;97:1440–1445. doi: 10.1161/01.CIR.97.15.1440. - PubMed
-
- Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

