Codon usage of highly expressed genes affects proteome-wide translation efficiency
- PMID: 29735666
- PMCID: PMC6003480
- DOI: 10.1073/pnas.1719375115
Codon usage of highly expressed genes affects proteome-wide translation efficiency
Abstract
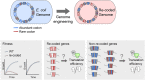
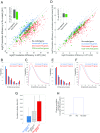
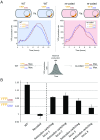
Although the genetic code is redundant, synonymous codons for the same amino acid are not used with equal frequencies in genomes, a phenomenon termed "codon usage bias." Previous studies have demonstrated that synonymous changes in a coding sequence can exert significant cis effects on the gene's expression level. However, whether the codon composition of a gene can also affect the translation efficiency of other genes has not been thoroughly explored. To study how codon usage bias influences the cellular economy of translation, we massively converted abundant codons to their rare synonymous counterpart in several highly expressed genes in Escherichia coli This perturbation reduces both the cellular fitness and the translation efficiency of genes that have high initiation rates and are naturally enriched with the manipulated codon, in agreement with theoretical predictions. Interestingly, we could alleviate the observed phenotypes by increasing the supply of the tRNA for the highly demanded codon, thus demonstrating that the codon usage of highly expressed genes was selected in evolution to maintain the efficiency of global protein translation.
Keywords: codon usage evolution; codon-to-tRNA balance; genome engineering; tRNA; translation efficiency.
Conflict of interest statement
Conflict of interest statement: G.M.C. is a co-founder of EnEvolv.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

