Long-distance electron transport in individual, living cable bacteria
- PMID: 29735671
- PMCID: PMC5984516
- DOI: 10.1073/pnas.1800367115
Long-distance electron transport in individual, living cable bacteria
Abstract
Electron transport within living cells is essential for energy conservation in all respiring and photosynthetic organisms. While a few bacteria transport electrons over micrometer distances to their surroundings, filaments of cable bacteria are hypothesized to conduct electric currents over centimeter distances. We used resonance Raman microscopy to analyze cytochrome redox states in living cable bacteria. Cable-bacteria filaments were placed in microscope chambers with sulfide as electron source and oxygen as electron sink at opposite ends. Along individual filaments a gradient in cytochrome redox potential was detected, which immediately broke down upon removal of oxygen or laser cutting of the filaments. Without access to oxygen, a rapid shift toward more reduced cytochromes was observed, as electrons were no longer drained from the filament but accumulated in the cellular cytochromes. These results provide direct evidence for long-distance electron transport in living multicellular bacteria.
Keywords: Raman spectroscopy; cable bacteria; conduction; cytochrome c; electromicrobiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
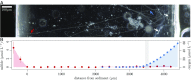
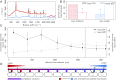

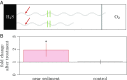
Comment in
-
Biological electron transport goes the extra mile.Proc Natl Acad Sci U S A. 2018 May 29;115(22):5632-5634. doi: 10.1073/pnas.1806580115. Epub 2018 May 16. Proc Natl Acad Sci U S A. 2018. PMID: 29769327 Free PMC article. No abstract available.
References
-
- Pfeffer C, et al. Filamentous bacteria transport electrons over centimetre distances. Nature. 2012;491:218–221. - PubMed
-
- Burdorf LDW, et al. Long-distance electron transport occurs globally in marine sediments. Biogeosciences. 2017;14:683–701.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

