High mobility group A2 (HMGA2) deficiency in pigs leads to dwarfism, abnormal fetal resource allocation, and cryptorchidism
- PMID: 29735702
- PMCID: PMC6003518
- DOI: 10.1073/pnas.1721630115
High mobility group A2 (HMGA2) deficiency in pigs leads to dwarfism, abnormal fetal resource allocation, and cryptorchidism
Abstract
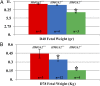
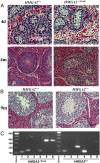
Expression of HMGA2 is strongly associated with body size and growth in mice and humans. In mice, inactivation of one or both alleles of Hmga2 results in body-size reductions of 20% and 60%, respectively. In humans, microdeletions involving the HMGA2 locus result in short stature, suggesting the function of the HMGA2 protein is conserved among mammals. To test this hypothesis, we generated HMGA2-deficient pigs via gene editing and somatic cell nuclear transfer (SCNT). Examination of growth parameters revealed that HMGA2-/+ male and female pigs were on average 20% lighter and smaller than HMGA2+/+ matched controls (P < 0.05). HMGA2-/- boars showed significant size reduction ranging from 35 to 85% of controls depending on age (P < 0.05), and organ weights were also affected (P < 0.05). HMGA2-/+ gilts and boars exhibited normal reproductive development and fertility, while HMGA2-/- boars were sterile due to undescended testes (cryptorchidism). Crossbreeding HMGA2-/+ boars and gilts produced litters lacking the HMGA2-/- genotype. However, analysis of day (D) D40 and D78 pregnancies indicated that HMGA2-/- fetuses were present at the expected Mendelian ratio, but placental abnormalities were seen in the D78 HMGA2-/- concepti. Additionally, HMGA2-/- embryos generated by gene editing and SCNT produced multiple pregnancies and viable offspring, indicating that lack of HMGA2 is not lethal per se. Overall, our results show that the effect of HMGA2 with respect to growth regulation is highly conserved among mammals and opens up the possibility of regulating body and organ size in a variety of mammalian species including food and companion animals.
Keywords: HMGA2; dwarfism; gene editing; organ size; swine.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973;38:14–19. - PubMed
-
- Xiang X, Benson KF, Chada K. Mini-mouse: Disruption of the pygmy locus in a transgenic insertional mutant. Science. 1990;247:967–969. - PubMed
-
- Hirning-Folz U, Wilda M, Rippe V, Bullerdiek J, Hameister H. The expression pattern of the Hmgic gene during development. Genes Chromosomes Cancer. 1998;23:350–357. - PubMed
-
- Chieffi P, et al. HMGA1 and HMGA2 protein expression in mouse spermatogenesis. Oncogene. 2002;21:3644–3650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

