Ultrafast glutamate sensors resolve high-frequency release at Schaffer collateral synapses
- PMID: 29735711
- PMCID: PMC6003469
- DOI: 10.1073/pnas.1720648115
Ultrafast glutamate sensors resolve high-frequency release at Schaffer collateral synapses
Abstract
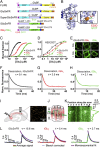
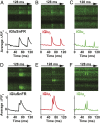
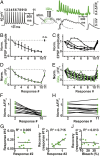
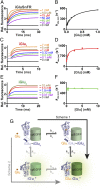
Glutamatergic synapses display a rich repertoire of plasticity mechanisms on many different time scales, involving dynamic changes in the efficacy of transmitter release as well as changes in the number and function of postsynaptic glutamate receptors. The genetically encoded glutamate sensor iGluSnFR enables visualization of glutamate release from presynaptic terminals at frequencies up to ∼10 Hz. However, to resolve glutamate dynamics during high-frequency bursts, faster indicators are required. Here, we report the development of fast (iGlu f ) and ultrafast (iGlu u ) variants with comparable brightness but increased Kd for glutamate (137 μM and 600 μM, respectively). Compared with iGluSnFR, iGlu u has a sixfold faster dissociation rate in vitro and fivefold faster kinetics in synapses. Fitting a three-state model to kinetic data, we identify the large conformational change after glutamate binding as the rate-limiting step. In rat hippocampal slice culture stimulated at 100 Hz, we find that iGlu u is sufficiently fast to resolve individual glutamate release events, revealing that glutamate is rapidly cleared from the synaptic cleft. Depression of iGlu u responses during 100-Hz trains correlates with depression of postsynaptic EPSPs, indicating that depression during high-frequency stimulation is purely presynaptic in origin. At individual boutons, the recovery from depression could be predicted from the amount of glutamate released on the second pulse (paired pulse facilitation/depression), demonstrating differential frequency-dependent filtering of spike trains at Schaffer collateral boutons.
Keywords: glutamate; hippocampus; synaptic transmission; two-photon imaging.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Best SL, Török K. Development of a fluorescent glutamate binding protein. Biophys J. 2005;88:46a.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

