Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche
- PMID: 29736022
- PMCID: PMC6095812
- DOI: 10.1038/s41591-018-0030-x
Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche
Erratum in
-
Author Correction: Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche.Nat Med. 2019 Apr;25(4):701. doi: 10.1038/s41591-019-0425-3. Nat Med. 2019. PMID: 30903101 Free PMC article.
Abstract
Aging of hematopoietic stem cells (HSCs) is associated with a decline in their regenerative capacity and multilineage differentiation potential, contributing to the development of blood disorders. The bone marrow microenvironment has recently been suggested to influence HSC aging, but the underlying mechanisms remain largely unknown. Here we show that HSC aging critically depends on bone marrow innervation by the sympathetic nervous system (SNS), as loss of SNS nerves or adrenoreceptor β3 signaling in the bone marrow microenvironment of young mice led to premature HSC aging, as evidenced by appearance of HSC phenotypes reminiscent of physiological aging. Strikingly, supplementation of a sympathomimetic acting selectively on adrenoreceptor β3 to old mice significantly rejuvenated the in vivo function of aged HSCs, suggesting that the preservation or restitution of bone marrow SNS innervation during aging may hold the potential for new HSC rejuvenation strategies.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
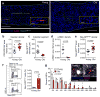
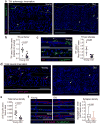
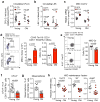
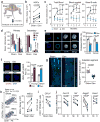

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

