Decreased IL-33 Production Contributes to Trophoblast Cell Dysfunction in Pregnancies with Preeclampsia
- PMID: 29736154
- PMCID: PMC5875049
- DOI: 10.1155/2018/9787239
Decreased IL-33 Production Contributes to Trophoblast Cell Dysfunction in Pregnancies with Preeclampsia
Abstract
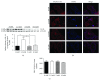
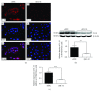
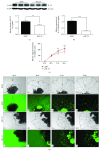
Preeclampsia (PE) is a life-threatening pregnancy complication which is related to aggradation of risk regarding fetal and maternal morbidity and mortality. Dysregulation of systemic inflammatory response and dysfunction of trophoblast cells have been proposed to be involved in the development and progression of PE. Some studies have demonstrated that interleukin-33 (IL-33) is an immunomodulatory cytokine that is associated with the immune regulation of tumor cells. However, little is known whether IL-33 and its receptor ST2/IL-1 R4 could regulate trophoblast cells, which are associated with the pathogenesis of PE. In this study, our target is to explore the impact of IL-33 on trophoblast cells and elucidate its underlying pathophysiological mechanisms. Placental tissues from the severe PE group (n = 11) and the normotensive pregnant women's group (n = 11) were collected for the protein expression and distribution of IL-33 along with its receptor ST2/IL-1 R4 via Western blot analysis and immunohistochemistry, respectively. We discovered that the level of IL-33 was decreased in placental tissues of pregnant women with PE, while no distinction was observed in the expression of ST2/IL-1 R4. These results were further verified in villous explants which were treated with sodium nitroprusside with different concentrations, to simulate the pathological environment of PE. To investigate IL-33 effects on trophoblast cells separately, IL-33 shRNA was introduced into HTR8/SVneo cells and villi. IL-33 shRNA weakened the proliferation, migration, and invasion capacity of HTR8/SVneo cells. The migration distance of villous explants was also markedly decreased. The reduced invasion of trophoblast cells is a result of IL-33 knockdown which could be related to the decline of MMP2/9 activity and the increased utterance of TIMP1/2. Overall, our findings demonstrated that the reduction of IL-33 production was connected with the reduced functional capability of trophoblast cells, thus inducing placental insufficiency that has been linked to the development of PE.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

