Pharmacokinetics, safety, and tolerability of the 2- and 3-direct-acting antiviral combination of AL-335, odalasvir, and simeprevir in healthy subjects
- PMID: 29736243
- PMCID: PMC5927802
- DOI: 10.1002/prp2.395
Pharmacokinetics, safety, and tolerability of the 2- and 3-direct-acting antiviral combination of AL-335, odalasvir, and simeprevir in healthy subjects
Abstract
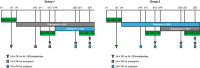
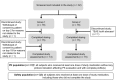
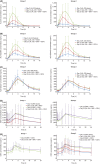
This Phase I, open-label, two-group, fixed-sequence study evaluated the pharmacokinetics and safety of AL-335, odalasvir, and simeprevir in healthy subjects. Group 1 (n = 16) received AL-335 800 mg once daily (QD) (days 1-3, 11-13, and 21-23), simeprevir 150 mg QD (days 4-23), and odalasvir 150 mg (day 14) followed by 50 mg QD (days 15-23). Group 2 (n = 16) received the same AL-335 regimen as in Group 1 plus odalasvir 150 mg (day 4) followed by 50 mg QD (days 5-23) and simeprevir 150 mg QD (days 14-23). Blood samples were collected to determine plasma concentrations of AL-335 (prodrug) and its metabolites, ALS-022399 (monophosphate precursor) and ALS-022227 (parent nucleoside), odalasvir, and simeprevir. Thirty-two subjects were enrolled. Odalasvir and simeprevir given alone, or in combination, increased AL-335 area under plasma concentration-time curve over 24 hours (AUC 0-24 h) 3-, 4-, and 7- to 8-fold, respectively; ALS-022399 AUC 0-24 h increased 2-, 2-, and 3-fold, respectively. Simeprevir had no effect on ALS-022227 AUC 0-24 h, whereas odalasvir with/without simeprevir increased ALS-022227 AUC 0-24 h 1.5-fold. AL-335 had no effect on odalasvir or simeprevir pharmacokinetics. Odalasvir and simeprevir AUC 0-24 h increased 1.5- to 2-fold for both drugs when coadministered irrespective of AL-335 coadministration. Study medications were well tolerated with no serious adverse events. One subject prematurely discontinued study drugs (unrelated event). This study defined the preliminary pharmacokinetic and safety profiles of the combination of AL-335, odalasvir, and simeprevir in healthy subjects. These data support the further evaluation of this combination for the treatment of chronic hepatitis C virus infection.
Trial registration: ClinicalTrials.gov NCT02512562.
Keywords: AL‐335; drug safety; drug‐drug interactions; hepatitis C virus; odalasvir; pharmacokinetics; simeprevir.
Figures
References
-
- World Health Organization . Hepatitis C fact sheet. 2017. http://www.who.int/mediacentre/factsheets/fs164/en/. (accessed 10 October 2017).
-
- American Association for the Study of Liver Diseases . HCV Guidance: Recommendations for testing, managing, and treating Hepatitis C. 2017. http://www.hcvguidelines.org/contents. (Accessed 9 October 2017)
-
- European Association for the Study of the Liver . EASL recommendations on treatment of hepatitis C 2016. J Hepatol. 2017;66:153‐194. - PubMed
-
- Gane EJ, Pianko S, Roberts SK, et al. Safety and efficacy of an 8‐week regimen of grazoprevir plus ruzasvir plus uprifosbuvir compared with grazoprevir plus elbasvir plus uprifosbuvir in participants without cirrhosis infected with hepatitis C virus genotypes 1, 2, or 3 (C‐CREST‐1 and C‐CREST‐2, part A): Two randomised, phase 2, open‐label trials. Lancet Gastroenterol Hepatol. 2017;2:805‐813. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

