Ultralong room temperature phosphorescence from amorphous organic materials toward confidential information encryption and decryption
- PMID: 29736419
- PMCID: PMC5935477
- DOI: 10.1126/sciadv.aas9732
Ultralong room temperature phosphorescence from amorphous organic materials toward confidential information encryption and decryption
Abstract
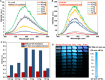
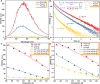
Ultralong room temperature phosphorescence (URTP) emitted from pure amorphous organic molecules is very rare. Although a few crystalline organic molecules could realize URTP with long lifetimes (>100 ms), practical applications of these crystalline organic phosphors are still challenging because the formation and maintenance of high-quality crystals are very difficult and complicated. Herein, we present a rational design for minimizing the vibrational dissipation of pure amorphous organic molecules to achieve URTP. By using this strategy, a series of URTP films with long lifetimes and high phosphorescent quantum yields (up to 0.75 s and 11.23%, respectively) were obtained from amorphous organic phosphors without visible fluorescence and phosphorescence under ambient conditions. On the basis of the unique features of URTP films, a new green screen printing technology without using any ink was developed toward confidential information encryption and decryption. This work presents a breakthrough strategy in applying amorphous organic materials for URTP.
Figures




References
-
- Pan Z., Lu Y.-Y., Liu F., Sunlight-activated long-persistent luminescence in the near-infrared from Cr3+-doped zinc gallogermanates. Nat. Mater. 11, 58–63 (2012). - PubMed
-
- Uoyama H., Goushi K., Shizu K., Nomura H., Adachi C., Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238 (2012). - PubMed
-
- Zhang Q., Li B., Huang S., Nomura H., Tanaka H., Adachi C., Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photonics 8, 326–332 (2014).
-
- Kishimura A., Yamashita T., Yamaguchi K., Aida T., Rewritable phosphorescent paper by the control of competing kinetic and thermodynamic self-assembling events. Nat. Mater. 4, 546–549 (2005). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

